Introduction
Lennox-Gastaut syndrome (LGS) is an electroclinical entity that is characterized by severe epileptic and developmental encephalopathy, which is associated with a high rate of morbidity and mortality and usually refractory to pharmacological treatment. Patients generally continue with seizures despite treatment with multiple antiseizure medicines (ASMs). Choosing suitable ASMs to maintain better seizure control, avoiding side effects and providing synergistic effects between drugs are crucial in management of LGS patients.
Case Report
A 9-year-old boy was brought for evaluation of seizures occurring since the age of 5 years. The patient was born at 38 weeks of gestation via normal vaginal delivery and had an uneventful course of birth. He had no history of perinatal distress, infection, encephalitis or exanthems. His first seizure at 5 years old was generalized tonic-clonic seizure (GTCS) and had been treated with phenobarbital 3 mg/kg/d at that time, and then was seizure free up to 1 year until parents noticed that the patient drops things from his hands. During the visit by a pediatric neurologist, carbamazepine 10 mg/kg/d was added to phenobarbital and the symptom disappeared and has not recurred up to 8 years old when other seizure types including many episodes of staring and occasional fallings started to emerge, and thereafter multiple ASMs had been tried to control patient’s seizures.
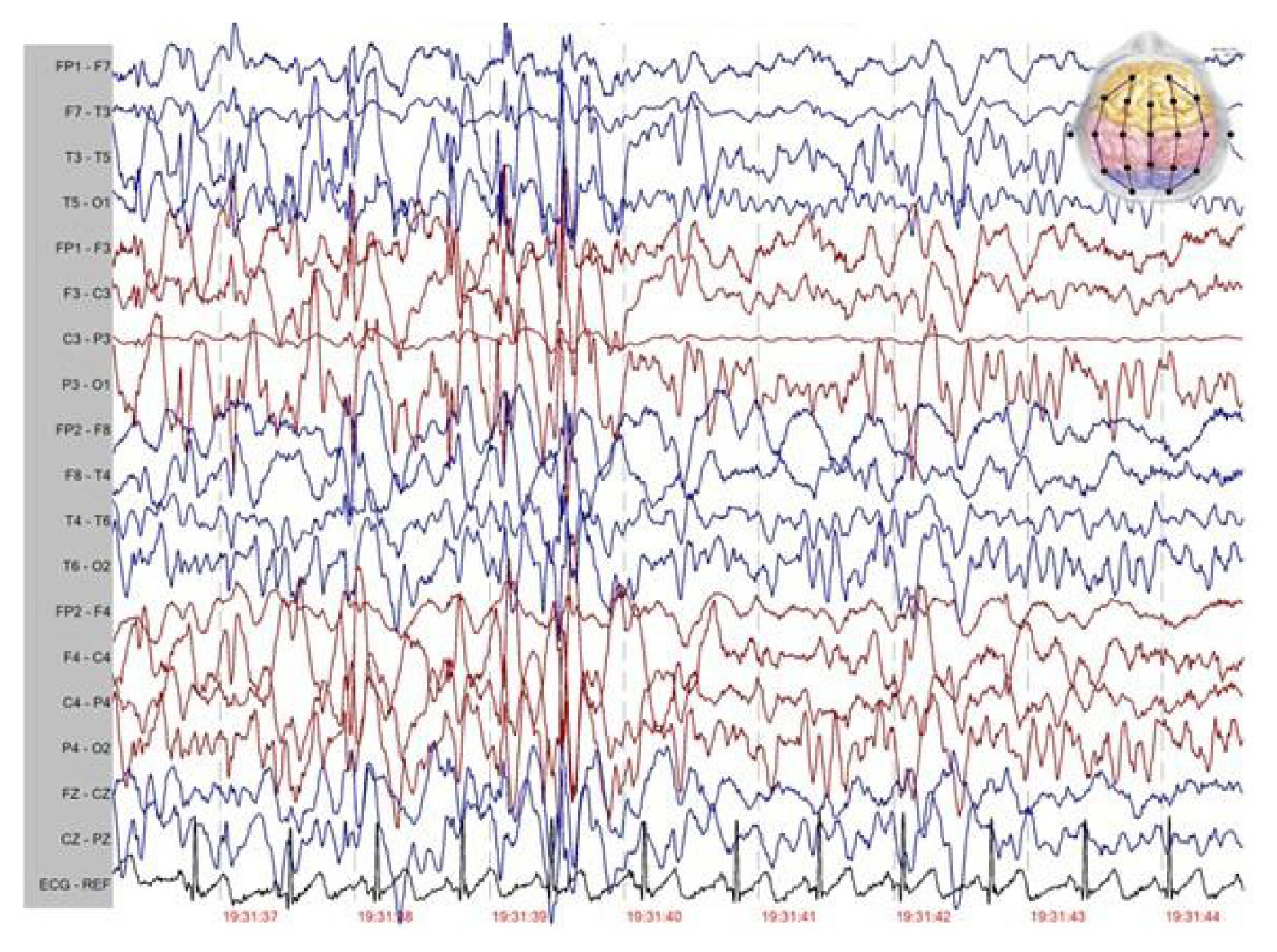
At the time of my visit, the patient had multiple seizure types including repetitive episodes of absence, GTCS, atonic and myoclonic seizures and was under treatment with vigabatrin 50 mg/kg/d, acetazolamide 20 mg/kg/d, and primidone 25 mg/kg/d. The patient also had learning disability and parents said that these drugs had been ineffective in control of seizures and spells continue to occur frequently each day. The patient was noted to suffer from many episodes of GTCS and to be resistant to ASMs had tried in the past. Atonic seizures were particularly troublesome and drop attacks had been causing repetitive facial and scalp lacerations and nasal fractures, but protective helmets had not been tolerated. His vitals were normal and laboratory data including complete blood count, basic metabolic panel, liver, and renal panel were within normal limits. Electroencephalography (EEG) revealed frequent bursts of generalized slow spike and wave discharges (1–2 Hz, 200–300 μV) with generalized paroxysmal fast activity plus to disorganized background consistent with EEG findings of LGS (Fig. 1). Brain magnetic resonance imaging (MRI) showed nothing of significance (Fig. 2). Genetic study was performed and was negative for abnormal genetic variants.
The patient’s medications were revised and topiramate was added to patient’s regimen, and he was sent home and advised of regular follow-up visits. Topiramate gradually increased up to 7 mg/kg/d, but there was little or no benefit, so clobazam 1.0 mg/kg/d was added again without remarkable benefit. Vigabatrin was slowly tapered due to lack of evidence in favor of tuberous sclerosis. Considering lack of response to these measures, the patient was referred to a tertiary center and his drugs changed to levetiracetam 50 mg/kg/d, primidone 25 mg/kg/d, and lamotrigine 3 mg/kg/d, but again seizures continued and finally a ketogenic diet was started for the patient. After starting the ketogenic diet, seizures frequency reduced, but after 2-month, patient was not compliant to the diet. As a measure of last resort, epileptic surgery was considered, and based on non lesional MRI, the patient underwent corpus callosotomy (Fig. 3). Callosotomy resulted in significant improvement, but after 4 months, the effect was diluted, and the patient’s seizure control was lost. The dosage of drugs was increased without meaningful effect.
Based on previous studies, lamotrigine combined with sodium valproate has evidence for synergies,1 so primidone was slowly cross tapered with addition of valproic acid. When the dosage of valproic acid reached to 25 mg/kg/d, I noticed significant reduction in seizure severity and frequency. Atonic seizures decreased to few times per week, and subsequently falling and injuries also decreased. As the seizure control was not complete, the dosage of lamotrigine increased by 12.5 mg every 2 weeks up to level of 5 mg/kg/d and at this point, all seizure types including absence seizures and drop attacks were almost disappeared and EEG that was obtained at that time showed almost complete disappearance of epileptic discharges (Fig. 4). The patient’s cognitive function and school performance were also significantly improved and after 2 years follow up, these effects are persisting and there’s only occasional staring episodes that are precipitated by febrile illnesses or insomnia.
Discussion
LGS is the most common form of epileptic encephalopathy. Most definitions of LGS require1 multiple seizure types,2 cognitive impairment, and the interictal EEG pattern termed slow spike wave (typically 1.0 to 2.5 Hz, usually generalized and occasionally multi-focal). 2 Antiepileptic treatment resistance is a rule in LGS and monotherapy often fails so that combination therapy often is inevitable. International League Against Epilepsy (ILAE) defines resistant epilepsy as failure of at least 2 trials of accurately selected and adequately dosed ASMs,3 so our patient meets the official ILAE criteria for drug-resistant epilepsy. As complete seizure freedom is rarely achieved in treatment of LGS, the principal goal of treatment is seizures control to prevent physical injuries, and to some extent, improvement of cognition. Among the seizure types in LGS, atonic seizures usually are most difficult to control, and this render the patient at risk for traumatic injury to the face, jaw, teeth, and brain. There is evidence for effectiveness of few medications such as rufinamide in controlling drop seizures in LGS, which is not available in our country, but also concerns about its hepatotoxicity limits its use.4 Current evidence favors the continued use of sodium valproate as the first-line treatment for patients with newly diagnosed de novo LGS. If sodium valproate is ineffective alone, evidence supports lamotrigine, or subsequently rufinamide as adjunctive therapy.5
Lamotrigine is approved in Europe and the USA for the treatment of seizures associated with LGS. Motte et al. showed that lamotrigine is effective and well tolerated in the treatment of LGS.6 As noted earlier, there is evidence for synergistic effect of valproic acid and lamotrigine combination therapy in resistant epilepsy, and many experts feel that the combination of lamotrigine with sodium valproate is one of the best for the treatment of LGS,7 so this fact applied to the patient.1,8 The novel concept in this LGS patient was dramatic response of drop seizures to this combination, as the drop attacks have been almost completely resolved. As the treatment of LGS and prevention of drop attacks remain a great concern for patients, addressing this concern with lamotrigine-valproic acid plus levetiracetam combination therapy maybe an option for practicing clinicians even in patients in whom corpus callosotomy fails.