Introduction
Aphasic status epilepticus (ASE) is an unusual condition and has clinical characteristics similar to other disorders. However, aphasia is the sole manifestation of seizure in patients with ASE; therefore, other symptoms of status epilepticus, such as consciousness alterations, behavioral and psychiatric symptoms are absent.1,2 Aphasic status epilepticus can emerge from various metabolic or neurological disorders.3–7 Herein, we report two cases of ASE associated with Alzheimer’s disease (AD) and discuss their clinical characteristics.
Case Report
Patient 1
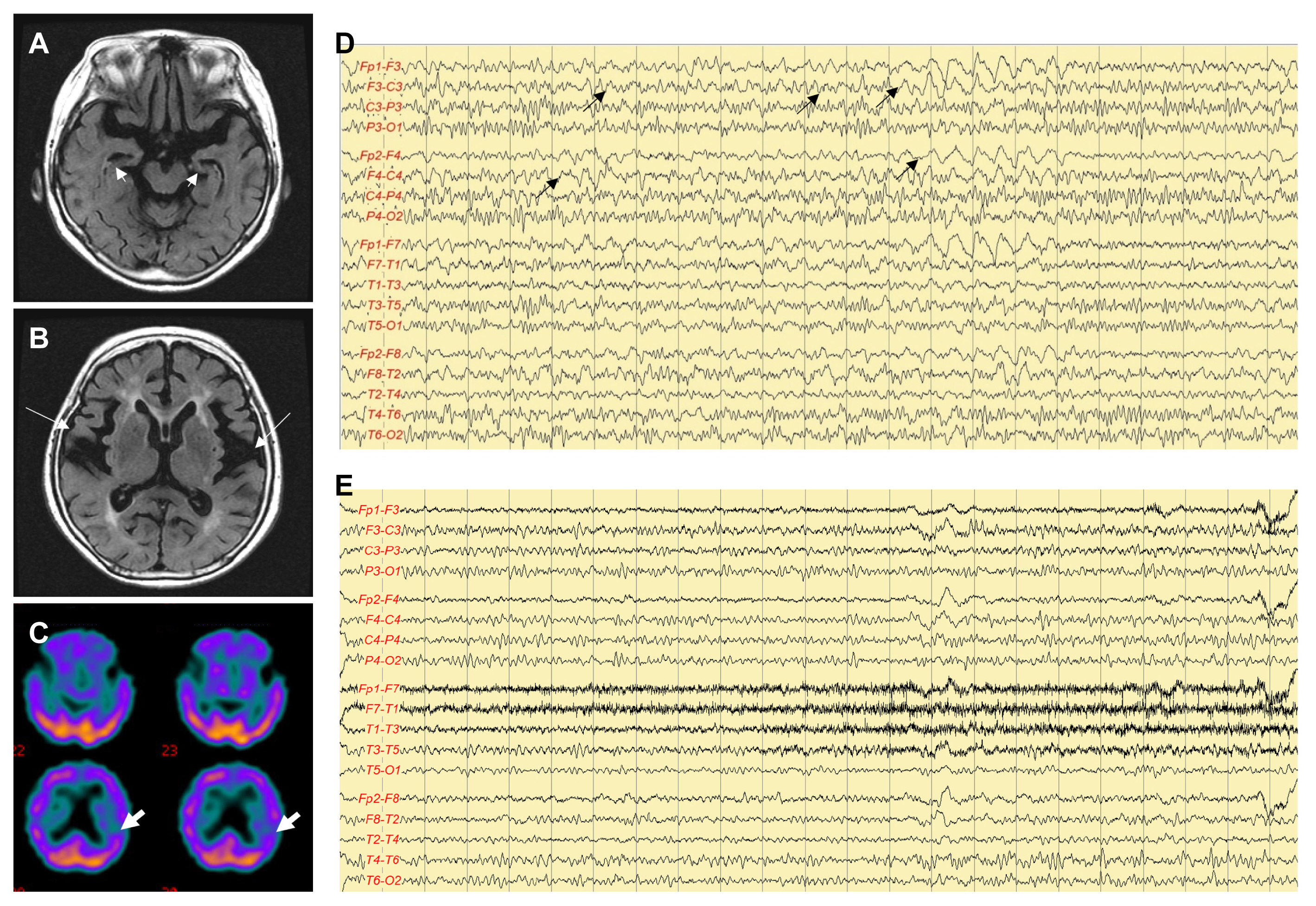
An 82-year-old right-handed woman developed speech disturbance a week before visiting the Department of Neurology. On neurological examination, the patient was alert and followed one-step verbal commands. However, she could not speak spontaneously. Physical examination revealed no abnormalities. Her medical history, as reported by her husband, revealed that she had experienced a mild decline in memory over the past year; however, she could speak and perform daily activities without any difficulty until a week before this visit. Routine laboratory tests revealed no abnormalities. Electroencephalogram (EEG) revealed an intermittent rhythmic delta activity and quasi-rhythmic fast activity in the frontotemporal area bilaterally, with fluctuating frequency and amplitude (Fig. 1D). The patient was aphasic during the EEG recording period. Brain magnetic resonance imaging (MRI) exhibited diffuse atrophy of the cerebral cortex and hippocampal formation (Fig. 1A, B). The symptoms and EEG abnormalities were absent a day after the administration of valproate (loading dose of 25 mg/kg, and maintenance doses of 6 mg/kg) (Fig. 1E). The patient reported remembering nearly all events during the seizure episodes. Brain single-photon emission computed tomography (SPECT) performed after the control of ASE revealed decreased perfusion in the left frontotemporal area (Fig. 1C). The patient score on the Korean version of the Mini-Mental Status Examination (K-MMSE) was 19 (0/3 in memory registration, 1/5 in attention and calculation, 0/1 in repetition, 4/5 in time orientation, and 3/5 in place orientation), and her Global Deterioration Scale (GDS) score was 4. A detailed neuropsychological test (Seoul Neuropsychological Screening Battery [SNSB]) presented global deterioration, particularly in the domains of memory registration and frontal executive function, which was compatible with AD. The patient follow-up revealed that she has been seizure-free for 5 years after discharge, and her cognitive function gradually has declined to a severe state of dementia, with K-MMSE and GDS scores of 3 and 6, respectively.
Patient 2
An 86-year-old right-handed woman developed sudden unresponsiveness and speech disturbances approximately 12 hours before visiting the Emergency Department. Her son reported that she was not able to understand him. She was diagnosed with AD 5 years before the visit to the clinic and has been irregularly treated with 10 mg of donepezil. The patient did not follow-up in the last 2 years before this visit. The patient scores at diagnosis were 15 on K-MMSE (0/3 in memory registration, 1/5 in attention and calculation, 2/3 in following commands, 0/1 in interlocking pentagon, 0/5 in time orientation, and 4/5 in place orientation) and 4 on GDS. The SNSB exhibited global deterioration, particularly in the domains of memory registration, language, and frontal executive function. On neurological examination, the patient was alert but could not follow verbal commands. However, she could look around and make eye-contact. Focal neurological abnormalities were not found. Laboratory test results revealed no abnormalities. Brain MRI revealed diffuse atrophy of the cerebral cortex and hippocampal formation without acute focal lesions (Fig. 2A, B). The initial EEG exhibited intermittent quasi-rhythmic theta and fast activity were observed bilaterally in the frontotemporal area with fluctuating frequency and amplitude (Fig. 2D). The patient remained aphasic throughout the recording period. The symptoms and EEG abnormalities were absent 8 hours after valproate administration (loading dose of 20 mg/kg, and maintenance doses of 6 mg/kg) (Fig. 2E). Brain SPECT performed after the control of ASE revealed decreased perfusion in the left frontal area (Fig. 2C). The follow-up K-MMSE and GDS scores were 10 and 5, respectively. The patient has been seizure-free for 6 months after discharge.
Discussion
Aphasia is a common neurological manifestation that rarely presents as seizures or status epilepticus; therefore, ASE has rarely been reported, although these reports found some variety in the type of aphasia (Broca’s, Wernicke’s, and global aphasia).8–11 In this study, patient 1 presented Broca’s aphasia, while patient 2 presented glob al aphasia.
None of the patients exhibited focal cerebral lesions. Focal lesions constitute most instances of ASE; however, nonfocal lesions have also been reported. In nonfocal lesions, metabolic disturbances, such as nonketotic hyperglycemia and aggravated uremia, or drugs, such as cefepime, are reportedly the cause of ASE.4–6,11 AD is a disorder with nonfocal lesions and can cause ASE.
The causes of seizures in AD are diverse, including extrasynaptic glutamate spillover; tau-induced enhancement of presynaptic glutamate release; reduced axonal and dendritic transport of mitochondria, which regulate neuronal excitability; selective impairment of gamma-aminobutyric acid-ergic interneurons in the hippocampus; altered amounts of voltage-gated ion channels in neurons; alterations in N-methyl-D-aspartate activity; shortened dendrites, which lowers the threshold for action potential generation; impaired cortical input to the reticular thalamic nucleus, which subsequently disinhibits thalamic relay nuclei; and increases in cholinergic tone before the degeneration of cholinergic pathways.12 Subclinical epileptiform activity is often detected in patients with AD, in whom the progression of cognitive decline is faster.12 In patients presenting cognitive fluctuation or rapidly progressive cognitive decline, epileptiform activity and silent seizures should be investigated by EEG.13 Seizures may occur at any stage of AD.14,15 The presenting symptom of AD was ASE in patient 1, while in patient 2 ASE occurred in an advanced stage of AD.
The EEG findings were borderline in both patients, leading to a possible nonconvulsive status epilepticus (NCSE), according to the Salzburg criteria.16 However, the clinical symptoms and electrographic abnormalities responded well to treatment with antiepileptic drugs, characterizing their compatibility with NCSE. Subclinical rhythmic EEG discharges in adults (SREDA) is a rare benign EEG pattern,17 which resembles ictal discharges. Misinterpretation of SREDA may lead to misdiagnosis of epilepsy. The electrographic features of SREDA are characterized as symmetrical, diffuse, rhythmic monomorphic theta waves with sharp contours, usually maximal on the posterior (parietal and posterior temporal) leads.18 The quasi-rhythmic waves observed in the EEG from patient 2 are similar to the patterns of SREDA, with the difference in the fact that the quasi-rhythmic waves are maximal on the frontal leads, showing fluctuations in amplitude and frequency, and are accompanied with fast activity. Moreover, clinical symptoms and EEG abnormalities were absent after the administration of antiepileptic medication.
Valproic acid showed good efficacy and favorable tolerance for status epilepticus in previous studies.19,20 Low dose valproic acid is known to be tolerable in elderly patients.21
Diverse etiologies may lead to ASE, which is usually caused by focal cerebral lesions and less frequently by nonfocal lesions etiologies such as AD. In these cases, ASE may develop in early or advanced stage of AD. The patterns of EEG are diverse, and a typical rhythmic evolutional pattern may not be observed. Alterations in clinical symptoms and EEG patterns after treatment are the key to diagnosis. Prompt diagnosis and treatment are critical to prevent prolonged speech disturbances, which may cause further consciousness dysfunction.