A Case of Perampanel Overdose Presenting with Respiratory Failure
Article information
Abstract
Perampanel is a novel antiepileptic drug that has been used as an adjunctive treatment for focal-onset seizures. No reports to date have documented respiratory suppression as a side effect of perampanel in adults. Herein, we report a 51-year-old man with focal epilepsy presented with type 2 respiratory failure after accidently consuming of 66 mg of perampanel. Clinicians should consider the possibility of respiratory compromise whenever a high dose of perampanel needs to be administered to patients.
Introduction
Perampanel is a novel antiepileptic drug that has been used as an adjunctive treatment for focal-onset seizures. It has a long half-life of 105 hours and an oral preparation at a maximum dose of 12 mg/day is considered safe.1 Clinical trials have shown that perampanel is generally well-tolerated despite inducing dose-dependent adverse events of the central nervous system, such as dizziness, somnolence, irritability, headache, and psychiatric problems.2 Also, there are two cases with respiratory depression as an adverse events of perampanel in children previously diagnosed with compromised respiratory function.3 However, no reports to date have documented respiratory suppression as a side effect of perampanel in adults. Herein, we report a case of perampanel overdose with respiratory failure.
Case Report
A 51-year-old man with focal epilepsy visited the emergency department with altered mentality and irritability. The patient had a history of chronic subdural hemorrhage and mental retardation. He originally received perampanel (6 mg/day) and levetiracetam (1,000 mg 2 day). However, we estimated that he had accidentally consumed 66 mg of perampanel 1 hour before visiting our hospital. His parents denied the possibility of seizure provocation, including sleep deprivation, malnutrition, and alcohol consumption. Initially, his blood pressure was as high as 192/92 mmHg, but other signs, including body temperature, heart rate, and respiratory rate, were within the normal range. He also presented with a comatose mentality and roving eye movements without convulsions. Laboratory findings confirmed mild hypercapnia (partial pressure of CO2 [pCO2], 51.3 mmHg) and hypoxemia (partial pressure of O2 [pO2], 55.7 mmHg), and chest radiography revealed no active lung lesion. Moreover, brain computed tomography revealed no acute structural lesions. Electroencephalography showed no epileptiform discharges. At 2.5 hours after arrival at our hospital, his respiratory function deteriorated with aggravation of hypercapnia (62.3 mmHg) and desaturation (saturation of percutaneous O2, 86%) despite O2 supply (Fig. 1A). Because he had type 2 respiratory failure, intubation was performed and the respiratory rate was completely controlled via mechanical ventilation in the range of 13 to 20 times/min in the intensive care unit. Dexmedetomidine and remifentanil was used to control irritability. His Glasgow coma scale (GCS) score had deteriorated because of the increased amount of remifentanil used for controlling his irritability on the first day of hospitalization (hospital day 1). Thereafter, perampanel was replaced with 1,350 mg/day of valproic acid and the dose of levetiracetam was increased from 1,000 mg/day to 2,000 mg/day. After hospital day 2.5, the GCS score gradually improved (Fig. 1B). During ventilator care, we measured his airway occlusion pressure during the first 100 ms (P0.1) as a parameter of the level of ventilatory support.4 On hospital day 3, P0.1 was around 1.5 cmH2O, which indicated breathing with minimal support from the mechanical ventilator. After reducing the amount of remifentanil and dexmedetomidine on hospital day 4, hypercapnia and hypoxemia improved in the follow-up arterial blood gas profile (pCO2, 44.0 mmHg and pO2, 79.3 mmHg, respectively) on using the mechanical ventilator in the continuous positive airway pressure mode. On hospital day 5, the patient completely recovered consciousness to a normal awake status without any respiratory support. The patient was subsequently discharged and no seizures or complaints of respiratory discomfort were observed at the follow-up outpatient clinic.
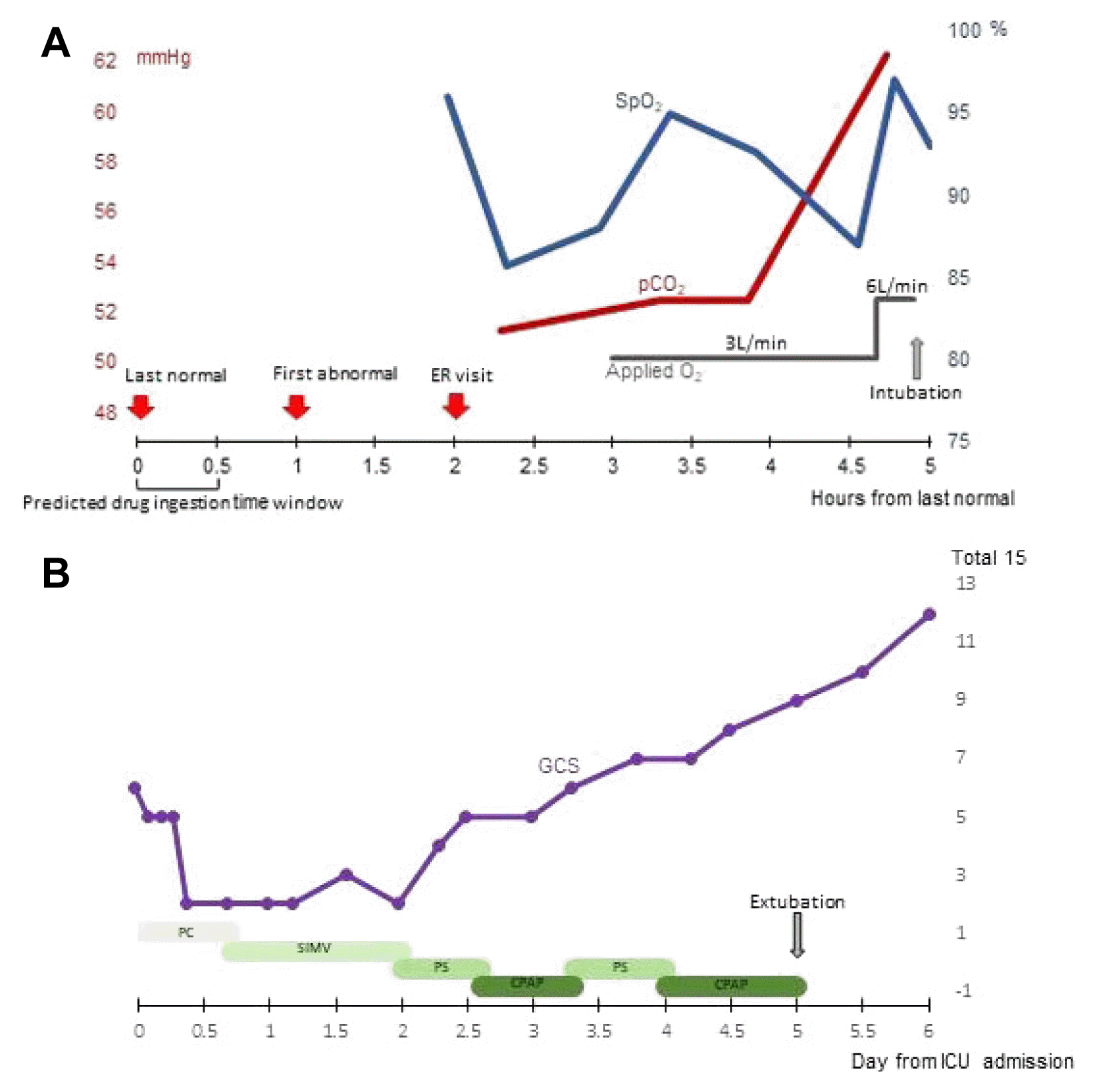
(A) Flow chart of arterial O2 and CO2 levels in the emergency room. (B) Flow chart of the mental status and supportive respiratory settings in the intensive care unit. Saturation of percutaneous oxygen (blue line); partial pressure of CO2, arterial partial pressure of carbon dioxide (red line); applied oxygen, applied non-invasive oxygen (gray line). ER, emergency room; ICU, intensive care unit; GCS, Glasgow coma scale; PC, pressure control ventilation mode; SIMV, synchronized intermittent mandatory ventilation mode; PS, pressure supportive ventilation mode; CPAP, continuous positive airway pressure mode.
Discussion
Perampanel is a noncompetitive antagonist of the postsynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.5 It induces various adverse events, such as irritability, aggression, and altered consciousness.6 However, evidence on the significant adverse effects of perampanel on vital signs, electrocardiography measures, and biochemical or hematologic parameters is currently lacking.7 Kim et al.8 reported the case of a 39-year-old woman with perampanel overdose (40 mg of perampanel) with a comatose mentality, but her respiratory function was normal. In another case, a man in his 20s with perampanel overdose (42 mg of perampanel) experienced prolonged unconsciousness, but showed no evidence of respiratory compromise.9 In children, there were two cases of respiratory depression with therapeutic dose of perampanel (0.04 mg/kg and 0.17 mg/kg) respectively, but they had compromised respiratory function before.3 In our case, perampanel overdose (1.05 mg/kg of perampanel) resulted in acute respiratory failure, which was not associated with other medical conditions. Moreover, unlike in these previous patients, our patient required mechanical ventilator support. Considering the half-life of perampanel is approximately 105 hours, respiratory suppression could be related to perampanel overdose. To the best of our knowledge, this is the first report of respiratory failure caused by acute perampanel overdose. Therefore, the possibility of respiratory compromise should be considered whenever a high dose of perampanel needs to be administered to patients.
Acknowledgements
We have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest. The authors report no disclosures.
Notes
The authors declare that they have no conflicts of interest.