Clinical Prediction Rule of Drug Resistant Epilepsy in Children
Article information
Abstract
Background and Purpose:
Clinical prediction rules (CPR) are clinical decision-making tools containing variables such as history, physical examination, diagnostic tests by developing scoring model from potential risk factors. This study is to establish clinical prediction scoring of drug-resistant epilepsy (DRE) in children using clinical manifestationa and only basic electroencephalography (EEG).
Methods:
Retrospective cohort study was conducted. A total of 308 children with diagnosed epilepsy were recruited. Primary outcome was the incidence of DRE. Independent determinants were patient characteristics, clinical manifestations and electroencephalography. CPR was performed based on multiple logistic regression.
Results:
The incidence of DRE was 42%. Risk factors were age onset, prior neurological deficits, and abnormal EEG. CPR can be established and stratified the prediction using scores into 3 levels such as low risk (score<6), moderate risk (score 6–12) and high risk (score>12) with positive likelihood ratio of 0.5, 1.8 and 12.5 respectively.
Conclusions:
CPR with scoring risks were stratified into 3 levels. The strongest risk is prior global neurological deficits.
Introduction
Epilepsy in children is a common problem which is treatable. Most uncomplicated patients can get remission with appropriate medications. However some patients are difficult to treat which is previously called pharmacoresistant or intractable epilepsy. According to revised definition of International League Against Epilepsy (ILAE) 2010, this term as mentioned is reconsidered to be called as “drug-resistant epilepsy (DRE)”. Its’ definition is “failure of adequate trial of two tolerated and appropriately chosen and used antiepileptic drugs (AED) schedules whether as monotherapy or combination to achieve sustained seizure freedom”.1 It must be helpful if there is a scoring model from risk factors for predicting DRE in order to plan for appropriate treatment and counselling. Many risk factors of DRE were reported such as age onset less than 1 year old, male, abnormal electroencephalography (EEG), neurological deficits.2–5 Clinical prediction rule (CPR) is a standardized clinical tool to stratify risk by scoring, help diagnosis and predict outcome.6,7 Establishment of CPR has 4 phases as follows: (1) development by identification of predictors, (2) internal and external validation, (3) impact analysis by measurement of cost-benefit, satisfaction, (4) implementation.8 The statistical models can accommodate many more factors and is capable of taking into consideration. This prediction model has been shown to be more accurate than clinical judgment alone. Scoring systems are usually derived from multiple regression analysis. Significant factors related to the outcome in observational studies are weighted as scores using lowest beta coefficient as baseline. For clinical application, the cumulative final scores are used as the indicators of the likelihood of outcome. The accuracy of CPR can be evaluated by area under the curve of receiver-operating characteristic (AUROC) curves which inform the percentage of accuracy explained by the model. The objective of this study is to establish a clinical prediction scoring of DRE in children.
Methods
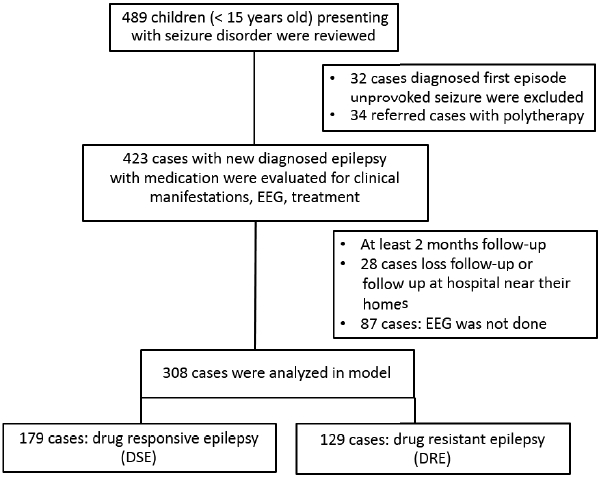
Retrospective cohort study was conducted. Study population was children diagnosed epilepsy treated with AEDs by ILAE definition as two or more unprovoked seizures at least 24 hours apart.2 Patients were followed up every 2–6 months at pediatric neurology clinic, regional Hatyai Hospital which is a referral center for seven general hospitals in southern part of thailand, during January 1998 to December 2012 (15 years) (Fig. 1). AED is given as initial monotherapy and evaluated every 2–6 months, at least 6 months after appropriate medication and dosage for seizure types. Primary outcome was the incidence of DRE by definition as mentioned earlier. The other is called as drug-responsive epilepsy (DSE) by the definition of achievement of seizure control with monotherapy. Independent determinants using patient characteristics as a principal model such as gender, underlying neurological deficits defined as motor deficits, mental deficits, global (motor and mental/psychosocial) deficits, clinical manifestations, plus only EEG as basic investigation were collected to determine risk factors. Neuroimaging were not considered in this model due to advanced investigation which did not an initial management and could not have been done as routine practice. Sample size was calculated using a formula of two independent groups and outcome as proportion. Incidence of epilepsy with and without risk factors of 50% and 20% respectively were used for sample size calculation.4 It will need to study at least 44 exposed subjects and 44 unexposed subjects to be able to reject the null hypothesis that the failure rates for experimental and control subjects are equal with 0.8 of power. The type I error probability associated with this test of this null hypothesis is 0.05. Data analysis were performed for score derivation based on multiple logistic regression. The scores were weighted by beta coefficient using the lowest regression coefficient equal 1 point as baseline and used it as the least common denominator for calculating each item’s score. The AUROC and likelihood ratio (LR) were also calculated after computing the predicted probability in order to stratify risk level. This study was approved by institutional review board for human research.
Results
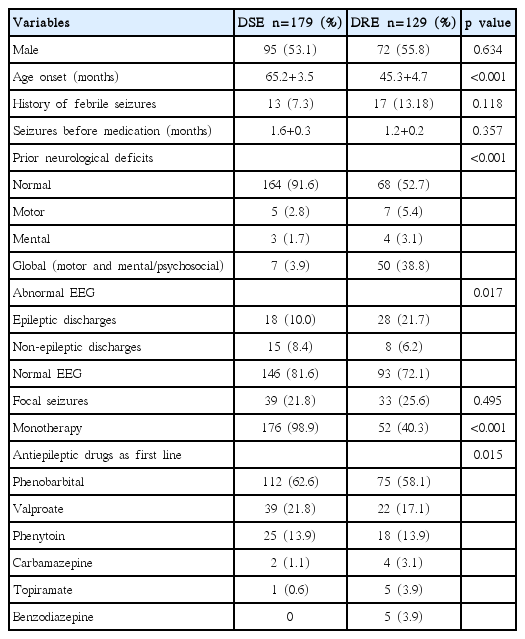
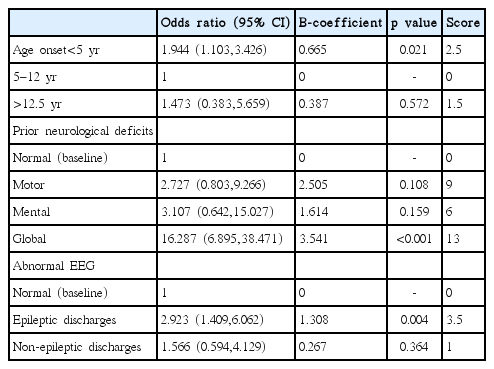
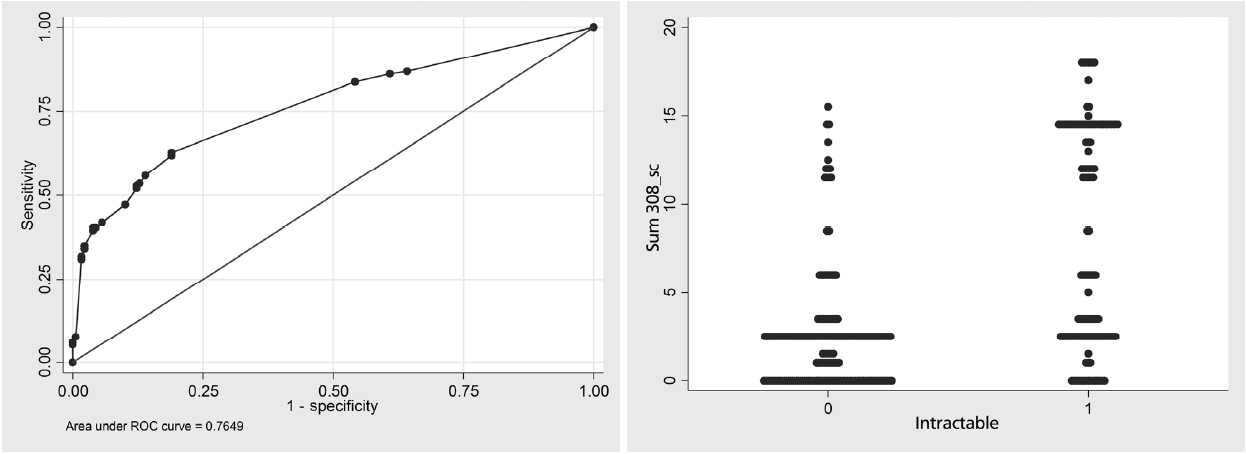
There were 308 cases recruited in this model analysis. Average age onset was 57 months old. The incidence of DRE was 129 cases (42%). Abnormal interictal EEG was found in 69 cases (22.3%) and focal seizures was diagnosed as 72 cases (23.4%). Most focal seizure was calssified by clinical semiology or EEG or history of focal ictal onset. Subtypes of generalized and focal seizures were not included in this study. Common first line AED were administered such as phenobarbital (60.7%), sodium valproate (14%) and phenytoin (9.8%). Comparison of patient characteristics between DSE and DRE are shown in Table 1. By multiple logistic regression analysis, risk factors were found such as age onset, prior neurological deficits, and abnormal interictal EEG. A model of CPR was performed for score derivation (Table 2). In order to explain the accuracy of this model, score distribution and area under the curve of ROC was calculated as high as 0.76 (Fig. 2). This CPR can be stratified into 3 levels by cut-off score as follows: low risk (score<6) with 50% of probability of DRE and +LR of 0.5, moderate risk (score 6–12) with 50–80% of probability of DRE and +LR of 1.8, and high risk (score>12) with more than 80% of probability of DRE and +LR of 12.5 (Fig. 3).
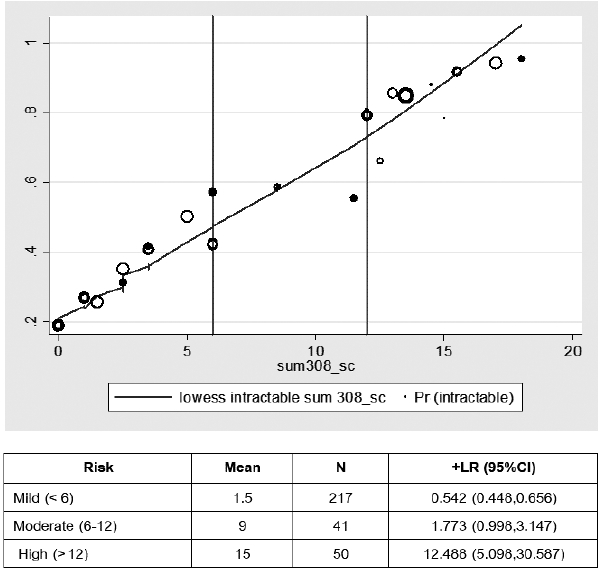
Stratification of risk score weighted by age onset into 3 groups. LR, likelihood ratio; CI, confidence interval.
Discussion
The incidence of DRE by revised definition of ILAE varies from 6 to 40% accordant with this study.1,3,9–12 This study was to create the clinical practice guideline of DRE in order to predict diagnosis and plan for counselling and management. The special statistical method is developed systematically based on best available evidences to make a clinical decision by transforming risk factors to scoring system.13 So, it can be widely used for not only experts but general practitioners, because it is just defined as a tool by quantify the contributions of history, clinical examinations, and basic diagnostic tests to stratify a patient in terms of the probability of diagnosis. Moreover, it can stratify risk level as low, moderate, and high which is convenient to apply in clinical practice. However, it is unclear if this reflects increasing usage of these tools in clinical practice or how this may vary across clinical areas. There is a study investigated whether published CPRs have been considered useful by expert and at the point of clinical care such as primary prevention of cardiovascular disease, diabetes mellitus screening, diagnosis or risk assessment, breast cancer diagnosis, screening, and risk assessment, depression diagnosis and management, acute childhood infections, namely meningitis, influenza, urinary tract infection, gastroenteritis, otitis media, tonsillitis, pneumonia, and bronchiolitis. General practitioners were surveyed about their use of CPRs in selected clinical areas. The results show main reasons for not using named CPRs related to lack of familiarity, preference for own clinical judgement, greater relevance to secondary care settings, and perceived lack of utility.14 This study is a preliminary report to help clinical decision making by CPR. It depends on administration of appropriate antiepileptic drugs and duration of effective assessment varying in clinical practice among experts. However clinical practice guidelines are updated routinely for initial monotherapy of seizure type and epileptic syndrome.2 Many risk factors of DRE were reported previously such as multiple seizures prior to treatment, focal seizures/complex seizures, age onset, developmental delay, abnormal EEG which are concordant with this report.15–17 All predictors of DRE were selected to establish a CPR in scoring system based on multiple logistic regression which is easy to interpret.18 There were 3 main predictors and can be separated into sub-items such as age onset, prior neurological deficits and abnormal EEG. Global neurological deficits was found as the strongest predictor. Nevertheless neuroimaging was done in only cases with indicated because we believed that obvious abnormal neuroimagings are associated with neurological deficits by physical examination. Limitation of this study is sample population recruited from only one referral center that could influence on external validity due to selection bias and referral bias. Moreover, children diagnosed benign childhood epilepsy with centrotemporal spikes confirmed by EEG but need AED according to parental concern with multiple seizures and juvenile myoclonic epilepsy known as very long-term medication dependence especially were also included.19,20
In conclusion, CPR of DRE is established and can be stratified scoring risk as 3 levels. The strongest risk is prior global neurological deficits.
Notes
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgements
The authors would like to thank Kriengsak Limpastan MD. Department of Neurosurgery, Faculty of Medicine, Chiangmai University for advice.