Terminology, incidence, and classification
Focal cortical dysplasia (FCD) is the most commonly encountered developmental malformation causing refractory epilepsy. Malformation of cortical development (MCD) is malformative lesions of the brain resulting from developmental aberration of normal processes that take place mostly during the first two trimesters of pregnancy and involving cells participating in the formation of the normal cerebral cortex. Neuronal migration disorder is regarded as an old terminology because not all MCDs are caused by neuronal migration anomaly. There are heterogeneous groups of focal or diffuse malformations depending on the causes and timing of the defect in the developmental processes.
The incidence of MCDs is variable according to the development and application of high resolution MRI. There is increasing tendency in the incidence of MCDs because of the advancement of this technique.1,2 It is the most common etiology of the cryptogenic and probable symptomatic epilepsy. It comprises 25 to 40% of the refractory childhood epilepsies.3 75% of patients with MCDs will have epilepsy at some time in their life.4
Both genetic and environmental factors play in the genesis of MCDs (Table 1)5. Recently, whole exom sequencing demonstrated that mutations of WD repeat domain 62 was the cause of wide spectrum of severe cortical malformation including microcephlay, pachygyria, lissencphaly, schizencephaly, polymicrogyria, and hypoplasia of corpus callosum.6 Another whole exom sequencing also identified de novo somatic mutations of PIK3CA, AKT3, and MTOR genes in focal brain areas.7
There is no general consensus on the classification of MCD because a particular defect in the cortical development may be responsible for more than one morphological subcategory of MCDs and one morphological subtype of MCD may arise from more than one mechanism for its formation. The most commonly used classification is Barkovich classification.8 It has four categories based on the major stages of brain development. They are malformation due to abnormal neuronal and glial proliferation, malformation due to abnormal neuronal migration, malformation due to abnormal cortical organization including later neuronal migration, and unclassified malformations. However, with the application of this system on the classification of FCD, FCD should be subdivided into two subcategories. FCD without balloon cells belongs to the category of malformation due to abnormal cortical organization including later neuronal migration and FCD with balloon cells belongs to the category of abnormal neuronal and glial proliferation.
FCD has intrinsic epileptogenicity because of the abnormal arrangement of the intralesional circuitry. The epileptogenic properties of dysmorphic neurons are well demonstrated in FCD. Ictal discharges and interictal spikes originated from FCD area where dysmorphic neurons were located.9 Seizures also started from the cortical area characterized by dysmorphic neurons but not balloon cells.10 Cytomegalic neurons demonstrated abnormal electrophysiological properties.11 There is imbalance between glutamergic excitatory and GABAergic inhibitory inputs.12,13 NR2A and NR2B subunits are increased in FCD area.14
Since the first distinctive and characteristic focal pathology was described,15 numerous classification schemes have been presented. Tassi et al16 proposed a new simplified classification based on the pathological characteristics with some other aspects including MRI findings and clinical considerations. They are architectural dysplasia, cytoarcitecural dysplasia, and Taylor-type cortical dysplasia. The common feature of these three entities was cortical laminar disruption. The distinction was based on the presence of cytological abnormalities such as giant neurons, dysmorphic neurons, and balloon cells.
Palmini et al17 further refined the terminology of classification of FCD with continuing criteria of histopatholgical findings. Recently ILAE proposed the new classification.18 In this system, FCD was classified into three entities (Table 2). The whole mark of Type I FCD is the cortical dyslamination and that of type II is the presence of dysmorphic neuron with or without balloon cells in addition to the cortical dyslamination. If there is another lesion other than type I or type II pathology, it is categorized into FCD type III.
Neuroimaging findings
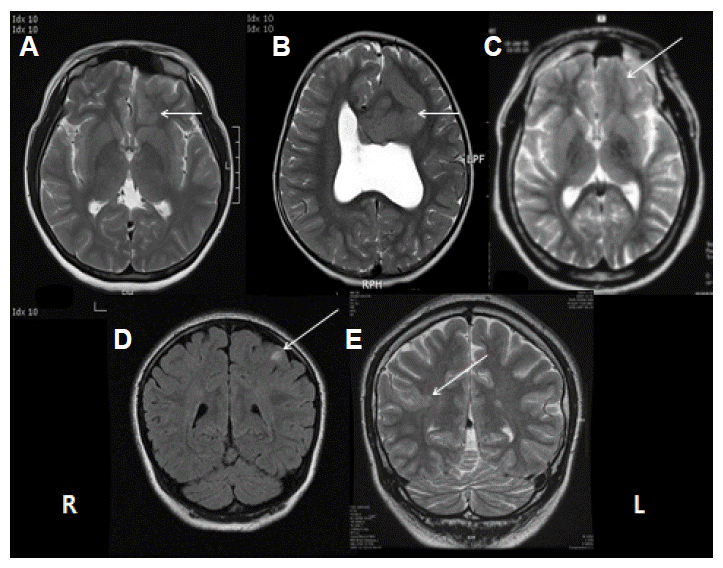
MRI features of FCD are variable. They are focal increase of cortical thickness, blurring of gray-white matter junction, increased signal in T2-weighted or FLAIR imaging, and increased signal from the cortical surface to the ventricle (Fig. 1). The final characteristic finding is called transmantle sign. Thin slice section and concomitant use of T2 and FLAIR imaging are important in detecting small FCD. The diagnostic sensitivity is increased along with the severity of pathology of FCD (Table 3).19 Thirty-seven percent of the patients with type I FCD had normal MRI while only 15% with FCD type II had negative MRI.16,19–22 The thick gray matter, blurring gray and white matter junction, and increased T2 or FLAIR signal are more frequently associated with type II FCD pathology. However, Most MRI findings could not differentiate Type I from Type II pathology because of significant overlapping of imaging findings between FCD type I and type II.23 Cortical thickness and blurring of GW junction were more common in isolated FCD than in FCD type III. And the transmantle sign on MRI was more specific to Type II.
The severer pathology might mean the higher epileptogenicity in FCD. However, the most severe pathological presentation can be found in the patients with negative MRI. The proportion of negative MRI in the patients with pathologically proven FCD ranges from 23% to 73%.24–26
Ictal SPECT and FDG-PET can be critical for localizing the seizure focus, especially in MR-negative patients. FDG-PET and subtraction SPECT are valuable in the diagnosis of MR-negative neocortical epilepsy.27–32 For focal cortical dysplasia, the diagnostic sensitivity of FDG-PET and ictal SPECT ranges from 69% to 98% and 48% to 64%, respectively.21,33–38
Epilepsy surgery of FCD
The proportion of FCD in surgical series ranges from 9% to 71% depending on the slection criteria of different centers.16,19,22,34–36,39–42 Our series40 included only neocortical epilepsy patients. In this series the proportion of FCD reached 71%. The relative poor outcome of FCD compared with benign brain tumor or temporal lobe epilepsy (TLE) with hippocampal sclerosis can be noticed. Whereas approximately 80% of patients became seizure free after surgical treatment for mesial TLE related to HS or lesional epilepsy such as primary brain tumor or vascular malformations,43,44 the efficacy of surgical treatment for FCD was consistently less favorable, with approximately 33–75% of individuals becoming seizure free.16,36,39,45 The relative poor prognosis can be explained by that FCD is often invisible on MRI which leads to the difficulty to define epileptogenic zone. We have to depend only on semiology, EEG, and functional neuroimaging. The other factor affecting the relative poor surgical outcome is that even after the removal of all visible lesions on MRI, residual microscopic lesion can still be epilepstogenic, which means that dysplastic tissues tend to be more extensive than is apparent on MRI.21,46 Evidence indicates that even patients with MRI abnormalities who have resective epilepsy surgery for FCD have worse surgical outcomes than those of patients who have surgery for other focal lesional epilepsy syndromes.40 The complete resection of FCD is invariably associated with better surgical outcome (Table 4).19,20,22,36
Although MRI increases our confidence in the localization of epileptogenic foci and allows surgical resection to be performed without preoperative invasive studies, our series47 demonstrated that complete resection guided by areas of ictal onset, persistent pathological delta slowing, >1/sec frequent spikes, and intermittent fast activity (HFO) detected by intracranial EEG was very useful leading to the complete resection even in the patients with negative MRI. The importance of complete resection indicates that seizures may originate from periphery of FCD as well as the center of FCD. There is an inherent difficulty in identifying the epileptogenic zone in MR-negative FCD patients, which often results in incomplete resection. However, with careful interpretation of other studies including functional neuroimaging and concordant results among these studies, surgical treatment can benefit patients with MR-negative FCD.
The positive prognostic effect of severe pathological features has been documented in previous reports, which predominantly included patients with identifiable lesions on MRI.2,16,45,48 Our series19 also identified that the patients with severe pathological features have a higher chance of becoming seizure free after surgery regardless of MRI results. The prognostic role of pathological severity may be related to the completeness of surgical resection. Although the epileptogenic zone in FCD does not always correspond to the area with the most severe pathological features, it is generally accepted that the epileptogenicity of FCD is usually associated with its pathological severity. From this point of view, the severe pathology may mean more confined and higher epileptogenic area. The severe pathology can also easily be identified by other neuroimaging techniques. Furthermore, mild and severe pathology may have different characteristics of epileptogenic network. In other words, numerous variable entities may be clustered in mild pathology.
Conclusions
FCD is the most common MCD which is a major cause of epilepsy. Most MCDs are epileptogenic: FCD has intrinsic epileptogenicity. Neuroimaging development, especially high resolution MRI, can increase the detection rate of MCD, which can make us treat the patients surgically. FDG-PET and ictal SPECT can be valuable in the localization of FCD, which may be especially useful in the patients with normal MRI. Surgical treatment is a option for refractory epilepsy with FCD and complete resection of FCD area is the major prognostic factor. Complete resection of early ictal onset zone and resection which includes the area with pathological delta waves and frequent interictal spikes predict good surgical outcome. Pathologic characteristics are important not only for the correct diagnosis but also for prediction of surgical prognosis.