The main concerns with epilepsy during pregnancy are the maternal and fetal risks from uncontrolled seizures and the fetal exposure to antiepileptic drugs (AEDs). In spite of the fact that most studies have shown that pregnancy does not seem to significantly change seizure control in most women, there are diverse effects of pregnancy on epilepsy, which are explained by altered hormonal levels, a changed drug metabolism, and many psychobehavioral factors, such as compliance, stress, and emotional changes [1]. Status epilepticus (SE) in pregnancy is very rare, and there have been only a few case reports of refractory SE (RSE) during pregnancy in patients with systemic lupus erythematosus (SLE), porphyria, vitamin B6 deficiency, and cavernous angioma [2,3]. We report on a case of RSE in a patient with epilepsy from cortical dysplasia. The RSE was not controllable with any AEDs or other treatments, and it spontaneously resolved after parturition.
Case Report
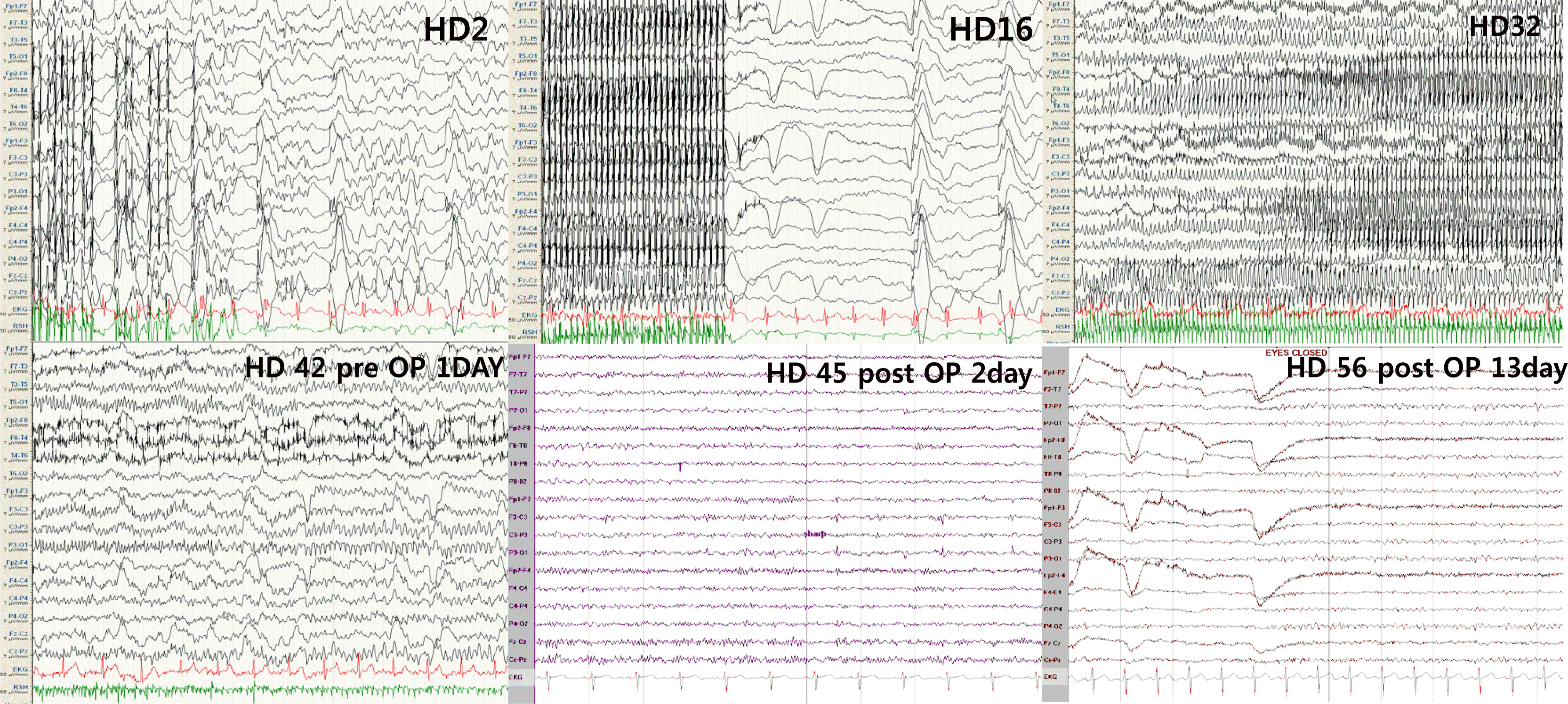
The patient was a 27-year-old primigravida who was diagnosed as epilepsy with cortical dysplasia 21 years before. Her previous seizures were reasonably controlled by topiramate, carbamazepine, and pregabalin. At 23 weeks of pregnancy, her seizure frequency progressively increased. She came to the ER in a confused mental state with intermittent convulsive movements in her arm that lasted for a few hours. On neurological examination, her mental status was stuporous, but there were no focal neurological deficits. Initial electroencephalography (EEG) revealed very frequent and sharp medium- to high-voltage waves in the left fronto-centro-parietal area that built up repetitive high-voltage, generalized sharp, and wave complexes for 10 to 20 sec, which suggested SE. These ictal rhythms were correlated with initial head and eyeball deviation that was secondary to generalized tonic-clonic seizures. Between the seizures, consciousness was not regained. Except for the pregnancy, there were neither precipitating factors nor laboratory abnormalities.
She was admitted to the ICU, and she was managed with a continuous infusion of intravenous midazolam under continuous EEG (c-EEG) monitoring. On the EEG, very frequent sharp waves with repetitive ictal rhythms appeared in the left centro-parieto-occipital area. Midazolam was titrated up to 5.5 mg/kg/h, but the ictal rhythms with clinical seizures were not successfully controlled. On the fifth hospital day (HD 5th), midazolam was dosed up to 17 mg/kg/h, her blood pressure was reduced to 80/50, but the repeated seizures were not controlled. We added 3,000 mg of levetiracetam per day. Midazolam was tapered slowly from HD 10th to HD 16th, and then her mental state and EEG findings were improved except for an occasional brief seizure. On HD 32nd, she was transferred to the ward with c-EEG monitoring. However, three days later, very frequent generalized seizures occurred again. Phenytoin, phenobarbital, and pentobarbital were sequentially loaded for a few days with intervals, but SE continued.
On HD 43rd, at 29 weeks of pregnancy, a cesarean section was performed. After the parturition, the EEG gradually improved. The EEG performed the day after parturition showed only intermittent median voltage sharp waves in the left centro-parietal area. Her mentality gradually improved, and accordingly, previous anti-SE drugs were gradually tapered out. She was discharged with an alert mental status, and the discharge medications were topiramate (300 mg), pregabalin (450 mg), levetiracetam (3,000 mg), and phenobarbital (180 mg). Her baby was raised in the newborn section because of premature and low birth weight. However, he did not show any other neonatal problems.
Discussion
SE during pregnancy is a relatively rare condition. According to a recent review of many studies of women with epilepsy, the rate of seizure increase during pregnancy ranged from 14% to 32%. The frequency of SE during pregnancy was 0% to 1.3%, which was less than the annual frequency of 1.6% for SE reported in a large series of patients with epilepsy [4,5]. These studies were not case-controlled, and so there is insufficient evidence to support an increased risk of SE and seizure frequency with pregnancy. However, we can confirm that the seizure threshold can be influenced by many factors during pregnancy, such as poor compliance and hormonal, pharmacokinetic, and emotional changes [1,6]. Therefore, SE in this state is not always predictable and preventable.
In our patient, the SE was not controllable by any antiepileptic drugs, including midazolam or pentobarbital, but it spontaneously ceased after parturition. She did not have a vitamin deficiency, metabolic abnormality, or any other medical problems. We considered that there might have been a different mechanism underlying the continual ictal activity and pharmacoresistancy, such as eclampsia. The precise etiopathogenesis of convulsions in eclampsia remains obscure, but recent studies have suggested that a different mechanism other than hypertension or cerebral edema can cause seizures in eclampsia. Neurotransmitters, such as neurokinin B and the proin-flammatory cytokines, TNF-a, IL-6, and IL-10, can excite NMDA glutamatergic receptors, and they play an important role in increasing neuronal excitability. They were reported to be increased in patients with eclampsia. Increased tissue plasminogen activator (t-PA), which activates excitatory receptors, may enhance epileptogenesis [7]. Nevertheless, our patient showed no signs of eclampsia or hormonal and neuromodulatory changes during pregnancy that might have contributed to the development of RSE. Unfortunately, the patient’s gestational age was too early for labor in our case, and so we had to wait, even though the best way to control eclampsia is with parturition as soon as possible. After parturition, the RSE of our patient was controlled immediately.
Above all, SE in the pregnant state is usually unpredictable and unpreventable. Thus, pregnancy in women with epilepsy needs to be well planned with stratified levels of management. In addition, parturition may be beneficial and the best treatment option in pregnant patients with RSE.