Introduction
Despite ongoing investigations of epilepsy and the development of several new antiepileptic drugs (AEDs), about a third of patients remain intractable.1,2 Recent exploration of new AEDs has focused on novel molecular targets, such as non-neuronal cells or neurotransmitter receptors, due to the strength of their effects on seizures and the role of para-neuronal cells in epilepsy.3–5 Perampanel (PER) is one of the newest drugs that targets glutamate excitatory neurotransmission through an α-amino-3-hydroxy-5-methyl-4-isoxazolepropinonic acid receptor. It is considered a better target than other glutamate-related receptors, because it contributes to fast synaptic excitation at the seizure onset.6 In a phase III clinical trial, PER showed a fair level of response rate among patients with focal and primary tonic-clonic seizures. Treatment-related adverse events were reported in more than 70% of patients taking 8 mg or higher dose of PER, but most of these were not severe.7–9 In particular, the responder rate was maintained during a 2-year follow-up extension study.10 Several clinical reports have been published since its release, but the efficacy profiles vary between studies from 27% to 53% and reported adverse events have been diverse and differ among age groups. Considering the diversity of patient ages and epileptic syndromes included, long-term pediatric data have been limited.11–13 Thus, we report our experiences with PER in adolescents and young adults with intractable focal epilepsy.
Methods
The study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 1802-052-921). We enrolled patients with intractable focal epilepsy, who were started on PER and had regular check-ups over 12 months at Seoul National University Children’s Hospital. Intractability was defined as failure to control seizures with adequate trials of two or more AEDs. Patients with generalized or undetermined seizure were excluded. We reviewed medical records retrospectively, including age, sex, seizure onset age, personal history of AEDs, results of laboratory and imaging tests, seizure frequency before and after PER administration, and newly occurred adverse events during PER treatment. Efficacy was evaluated based on the proportion of patients who were responders (i.e., who showed 50% or more seizure reduction), comparing the average number of seizures during the 28 days between baseline and maintenance periods. The baseline period was defined as the 12 weeks prior to first PER administration, and AEDs were maintained at the same dose during this period. The maintenance period was defined as total treatment duration after completion of 16 weeks of PER titration, although the actual titration schedule varied every 4–8 weeks in 71% of patients, and every 2–3 weeks in 20% (remaining patients discontinued PER at 2 mg/day). The final dose of PER was determined by the physician, considering the efficacy and tolerability. Consequently, efficacy data were obtained from patients consuming PER for more than 16 weeks, the titration period by definition. Tolerability was evaluated based on the retention rate and profile of adverse events. The retention rate at 3, 6, 12, and 18 months was calculated, and all patients were asked about any adverse events, including dizziness, ataxia, somnolence, fall, seizure aggravation, weight change, and psychological problems during PER use at every outpatient clinic visit. Psychological adverse events included aggression, behavioral problem, excessive irritability, suicidal idea, and significant mood changes. Statistical analyses were conducted using IBM SPSS Statstics 22 software suite (IBM SPSS, Chicago, IL, USA) with Student’s t-test for continuous variables and Pearson’s chi-square test for categorical variables. Statistical significance was considered at p < 0.05.
Results
Patient characteristics
Demographic data of the 97 enrolled patients are presented in Table 1. The first seizure occurred at an average age of 5.2 years (range, 0–15.4), and patients were treated with AEDs for approximately 13 years (range, 1.5–25.4 years). Forty patients (41.2%) were prescribed PER for the first time before 18 years of age, while the others (58.8%) began PER between 18–30 years of age. The mean follow-up duration after PER treatment was 15.9 months (range, 12–20 months) with a mean duration of treatment with PER of 10 months (range, 0–19 months). All patients had focal epilepsy with variable etiology. One-third of the patients (36.1%) had cryptogenic focal epilepsy without any structural lesion detected on the brain magnetic resonance imaging, whereas the others (63.9%) had structural lesion including congenital anomaly and acquired injury. The most common structural abnormality was focal cortical dysplasia and post-infectious lesion. Enrolled patients typically had highly intractable seizure. About half the patients (45, 46.4%) were on four or more concomitant AEDs. Epilepsy surgery was conducted on 22 patients (22.5%) and vagus nerve stimulation (VNS) was simultaneously used by 14 (14.3%) during PER administration.
Efficacy profiles and predictors
Efficacy was analyzed using seizure reduction records in 72 patients who were prescribed PER for 16 or more weeks. Titration schedule varied depending on the patient, but usually was every 4 or 8 weeks. Mean maintenance dose of PER was 6.6 mg (range, 2–12 mg), and responder rate was 41.7% (30/72 patients) on PER treatment. Among these, over 75% of seizure reduction was achieved in 11 patients (15.3%), including three seizure-free cases (4.2%). Responders accounted for 16 of the 34 patients (47.1%) in the adolescents group (ages 12–18 years) and 14 of 38 (36.8%) in the young adults group (ages 18–30 years). More than 50% seizure reduction was observed in nine out of 22 patients with cryptogenic focal epilepsy (40.9%), while 21 of 50 patients (42.0%) with structural lesions showed this rate of reduction. We compared clinical profiles of responders and non-responders to predict the fair efficacy of PER (Table 2). There were no significant clinical predictors of better response. However, higher number of concomitant medications, which indicated intractability of seizures, was likely to reduce the responder rate. The patients who achieved seizure-free status were 15.7, 19.1, and 22.4 years of age during PER initiation. All three patients had structural etiology with two or three concomitant AEDs. Their baseline seizure frequencies were 12, 10, and 0.7 times per 28 days, respectively, and their seizures decreased to zero with a maintenance dose of 4 mg/day. All three patients maintained a seizure-free status for over 12 months (one case for 14 months, others for 16 months).
Retention rate and tolerability profiles
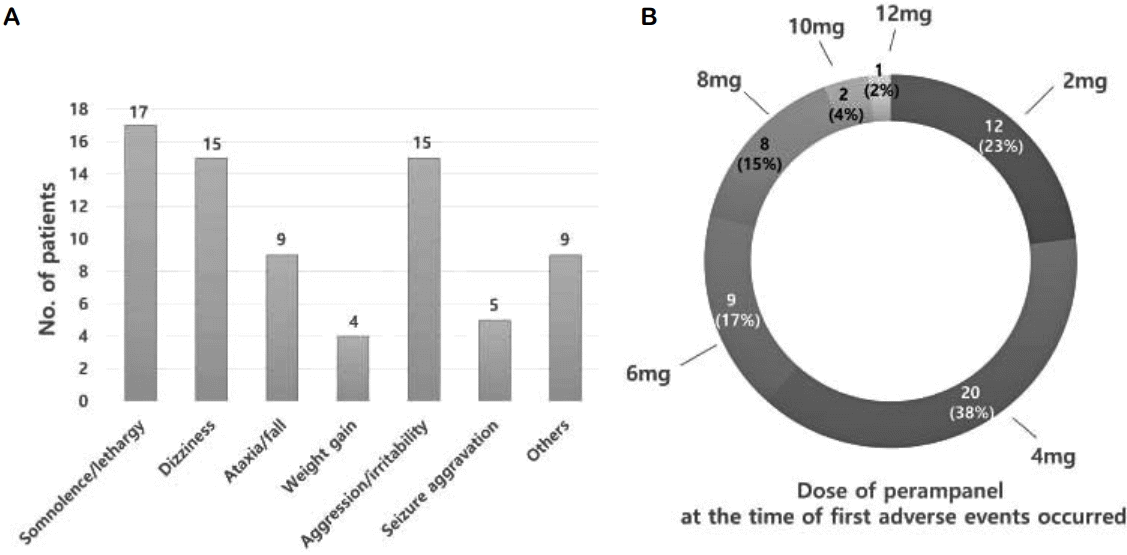
The retention rates at 3, 6, 12, and 18 months were 82.5% (80/97), 72.1% (70/97), 60.8% (59/97), and 37.5% (6/16), respectively. Forty-four patients (44/97, 45.4%) discontinued PER before their last visit. Among these, 20 patients (20/97, 20.6%) stopped the medication due to adverse events, while others (24/97, 24.7%) discontinued due to lack of efficacy. Fifty-two patients (53.6%) reported 74 types of adverse events (Fig. 1A), with somnolence or lethargy being the most common and reported in 17 patients (17/97, 17.5%). Dizziness and ataxia accounted for 15.3% and 9.3% of total patients, respectively, where three patients fell and had related trauma because of severe dizziness or ataxia. Psychological problems such as aggression or excessive irritability were other major adverse events observed in 15 patients (28%). Mood changes including depression or anxiety were not observed. Seizure aggravation was reported in five patients (5/97, 5.2%), who took PER for 0–3 months with a maximum dose of 4–6 mg/day. One of these patients showed a prolonged seizure few days after PER add-on. Over 60% of patients (32/52, 62%) reported their treatment-related adverse events at a PER dose of 4 mg/day or less (Fig. 1B). In particular, 12 patients experienced symptoms at the initiation dose of 2 mg/day, while 17 patients who once had some adverse event maintained the PER until their last clinic visit with fair efficacy. Among them, four patients reported improvement of symptoms after dose reduction and the rest adjusted with the same dose.
Discussion
Our cohort represented patients with severely intractable epilepsy. This has been a rare characteristic in other cohorts, particularly of adolescents.12,14 About 50% of our patients were on four or more AEDs, and epilepsy surgery and VNS were performed in 22 (22.7%) and 14 patients (14.4%), respectively. Average seizure frequency during the baseline period was 26.3 per 28 days. Nevertheless, PER showed a fair level of efficacy with a responder rate of 41.7%. According to previous real world retrospective studies, responder rates ranged from 19% to 50% and our data showed relatively high responder rate.11,14,15 Efficacy in the present cohort might be overestimated because 21 patients who discontinued PER within four months were excluded. Among these, however, 15 patients (71.4%) discontinued the medication due to treatment-related adverse events regardless of effectiveness, and six were prescribed 2 mg/day of PER until discontinuation. Therefore, any bias was most likely limited, and our data clearly supported the effectiveness of PER as an add-on therapy in patients with severely intractable focal seizures. As described in Table 2, neither intellectual disability nor age of PER initiation was associated with higher effectiveness. Other variables too did not show significant differences (p < 0.05), but the large number of adverse events were likely to have association with lower efficacy (p < 0.1). It represented intractability of seizures, which have been well known as an important factor for reducing the effectiveness of additive AED. Patients with four or more concomitant AEDs showed a 28.6% responder rate, and PER could be one treatment option in these patients. Further studies of PER efficacy in patients with detailed epilepsy syndrome, etiology, or other combined conditions are needed.
The proportion of adverse events and related medication withdrawal varies with studies, even for similar age groups.12–14,16 In the present study, adverse events and related AED discontinuation accounted for 53.6% and 20% of the cohort, respectively, both of which were relatively high. We also recognize that many adverse events reported were during administration of a low dose. This was inconsistent with previously established relation between dose and side effects.9 The average dose of PER during adverse events was 3.9 mg/day, despite the mean maximum dose of 6.9 mg/day in the present study. Twelve patients (12/52, 23%) reported adverse events at 2 mg/day dosage within few days of PER initiation. We can infer from this that a high burden of concomitant AEDs influenced these events, and clinically it is clear that careful observation is necessary from the initial use of PER in patients with multiple AEDs. Among such adverse events, psychiatric problems including aggression, irritability, anxiety, and mood instability have been noted from PER clinical trials. These appear to be more frequent among younger populations and among patients with prior psychiatric problems.16–18 However, Snoeijen-Schouwenaars et al.19 have insisted that pre-existing behavioral problems or polypharmacy are un-associated with high psychiatric adverse events. In our study, 15 patients (15.5%) showed aggression, irritability, or other behavioral problems at an average dose of 5.1 mg/day. Nine patients presented symptoms at a dose of 4 mg or less of PER. All except two patients finally discontinued the PER due to their adverse events. A 16-year-old male patient with three concomitant AEDs actually attempted suicide 2 months after PER administration (2 mg/day dose) and was admitted to a psychiatric ward. His symptoms completely disappeared after PER discontinuation. Among the patients with psychiatric adverse events, three patients (3/15, 20%) had pre-existing psychiatric problems, whereas only six (6/82, 7.3%) in that group were without psychiatric adverse events, although this was not statistically significant (p = 0.193). Intellectual disability was noted in seven and 31 patients (46.7% and 37.8%) who were with and without psychiatric adverse events, respectively (p = 0.462). Furthermore, age did not show any significant association with occurrence of adverse events (p = 0.471), although our cohort ranged from 12 to 30 years of age. The retention rate remained above 60% till 12 months of follow-up, but rapidly decreased to 37.5% at 18 months. In our cohort, all patients were monitored over 12 months, but 14 were monitored over 18 months after PER administration. Therefore, the retention rate at 18 months had low reliability and needed more follow-up. Our study also had some limitations that are commonly observed in other retrospective studies. The protocol for drug administration including initiation and titration schedule was not clearly established, and adverse events were identified by retrospective record reviews. Longer data collection in larger number of patients might be necessary for further validation of PER in clinical setting.
In conclusion, PER showed a fair level of efficacy in the present study, and it could be considered as a treatment option for intractable focal epilepsy. However, adverse events including psychiatric problems were very frequent even at a low dose. Therefore, pre-explanation about treatment-related adverse events and close monitoring at clinic visits is necessary.