1. Brodie MJ, Barry SJE, Bamagous G, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54.
2. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77.
3. French JA, Schachter SC, Sirven J, Porter R. The Epilepsy Foundation’s 4th Biennial Epilepsy Pipeline Update Conference. Epilepsy Behav. 2015;46:34–50.
4. Perucca E. Current trends in antiepileptic drug therapy. Epilepsia. 2003;44:Suppl 4. 41–7.
5. Mintzer S, Mattson RT. Should enzyme-inducing antiepileptic drugs be considered first-line agents? Epilepsia. 2009;50:Suppl 8. 42–50.
6. Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54:11–27.
7. Deckers CL, Hekster YA, Keyser A, van Lier HJ, Meinardi H, Renier WO. Monotherapy versus polytherapy for epilepsy: a multicenter double-blind randomized study. Epilepsia. 2001;42:1387–94.
8. Barker-Haliski M, Sills GJ, White SH. What are the arguments for and against rational therapy for epilepsy? Adv Exp Med Biol. 2014;813:295–308.
9. Lee BI, No SK, Yi SD, et al. Unblinded, randomized multicenter trial comparing lamotrigine and valproate combination with controlled-release carbamazepine monotherapy as initial drug regimne in untreated epilepsy. Seizure. 2018;55:17–24.
10. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9.
11. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurology. 2018;75:279–86.
12. Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62:375–81.
13. Canevini MP, De Sarro G, Galimberti CA, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797–804.
14. Luoni C, Bisulli F, Canevini MP, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52:2181–91.
15. Lammers MW, Hekster YA, Keyser A, Meinardi H, Renier WO, van Lier H. Monotherapy or polytherapy for epilepsy revisited: a quantitative assessment. Epilepsia. 1995;36:440–6.
16. French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50:Suppl 8. 63–8.
17. Brodie MJ, Sills GJ. Combining antiepileptic drugs--rational polytherapy? Seizure. 2011;20:369–75.
18. Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9:464–8.
19. Lasoń W, Dudra-Jastrzębska M, Rejdak K, Czuczwar SJ. Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamics interactions: an update. Pharmacol Rep. 2011;63:271–92.
20. Kaminski RM, Matagne A, Patsalos PN, Klitgaard H. Benefit of combination therapy in epilepsy: a review of the preclinical evidence with levetiracetam. Epilepsia. 2009;50:387–97.
21. Besag FM, Berry DJ, Pool F, Newbery JE, Subel B. Carbamazepine toxicity with lamotrigine: pharmacokinetic or pharmacodynamic interaction? Epilepsia. 1998;39:183–7.
22. Barcs G, Walker EB, Elger CE, et al. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia. 2000;41:1597–607.
23. Sake JK, Hebert D, Isojärvi J, et al. A pooled analysis of lacosamide clinical trial data grouped by mechanism of action of concomitant antiepileptic drugs. CNS Drugs. 2010;24:1055–68.
24. Margolis JM, Chu BC, Wang ZJ, Copher R, Cavazos JE. Effectiveness of antiepileptic drug combination therapy for partial-onset seizures based on mechanisms of action. JAMA Neurol. 2014;71:985–93.
25. Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 study group. Epilepsy Res. 1997;26:423–32.
26. Pisani F, Oteri G, Russo MF, Di Perri R, Perucca E, Richens A. The efficacy of valproate-lamotrigine comedication in refractory complex partial seizures: evidence for a pharmacodynamic interaction. Epilepsia. 1999;40:1141–6.
27. Kanner AM, Frey M. Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology. 2000;54:588–91.
28. McCabe PH, NcNew CD, Michel NC. Effect of divalproex-lamotrigine combination therapy in frontal lobe epilepsy. Arch Neurol. 2001;58:1264–68.
29. Moeller JJ, Rahey SR, Sadler RM. Lamotrigine-valproic acid combination therapy in medically refractory epilepsy. Epilepsia. 2009;50:475–79.
30. Rowan AJ, Meijer JW, de Beer-Pawlikowski N, van der Geest P, Meinardi H. Valproate-ethosuximide combination therapy for absence seizures. Arch Neurol. 1983;40:797–802.
31. Stephen IJ, Sills GJ, Brodie MJ. Lamotrigine and topiramate may be useful combination. Lancet. 1998;351:958–59.
32. Brigo F, Ausserer H, Tezzon F, Nardone R. When one plus one makes three: the quest for rational antiepileptic polytherapy with supraadditive anticonvulsant efficacy. Epilepsy Behav. 2013;27:439–42.
33. Chung S, Ben-Menachem E, Sperling MR, et al. Eamining the clinical utility of lacosamide: pooled analysies of three phase II/III clinical trials. CNS Drugs. 2010;24:1041–54.
34. Brodie MJ. Pharmacological treatment of drug-resistant epilepsy in adults: a practical guide. Curr Neurol Neurosci Rep. 2016;16:82
35. Kinirons P, McCarthy M, Doherty CP, Delanty N. Predicting drug-resistant patients who respond to add-on therapy with levetiracetam. Seizure. 2006;15:387–92.
36. Legge AW, Detyniecki K, Javed A, et al. Comparative efficacy of unique antiepileptic drug regimens in focal epilepsy: an exploratory study. Epilepsy Res. 2018;142:73–80.
37. Poolos NP, Warner LN, Humphreys SZ, Williams S. Comparative efficacy of combination drug therapy in refractory epilepsy. Neurology. 2012;78:62–8.
38. Chiron C, Marchand MC, Tran A, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomized placebo-controlled syndrome-dedicated trial. Lancet. 2000;356:1638–42.
39. Machado VH, Palmini A, Bastos FA, Rotert R. Long-term control of epileptic drop attacks with the combination of valproate, lamotrigine, and a benzodiazepine: a ‘proof of concept,’ open label study. Epilepsia. 2011;52:1303–10.
40. Dalic L, Cook MJ. Managing drug-resistant epilepsy: challeges and solutions. Neuropsychiatr Dis Treat. 2016;12:2605–16.
41. Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50:Suppl 8. 57–62.
42. Choi H, Hayat MJ, Zhang R, et al. Drug-resistant epilepsy in adults: outcome trajectories after failure of two medications. Epilepsia. 2016;57:1152–60.
43. Berg AT, Levy SR, Testa FM, D’Souza R. Remission of epilepsy after two drug failures in children: a prospective study. Ann Neurol. 2009;65:510–97.
44. Wirrell EC, Wong-Kisiel LC, Mandrekar J, Nickels KC. What predicts enduring intractability in children who appear medically intractable in the first 2 years after diagnosis? Epilepsia. 2013;54:1056–64.
45. Santulli L, Coppola A, Balestrini S, Striano S. The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol Res. 2016;107:211–9.
46. Shih JJ, Whitlock JB, Chimato N, Vargas E, Karceski SC, Frank RD. Epilepsy treatment in adult and aolescents: expert opinion, 2016. Epilepsy Behav. 2017;69:186–222.
47. Naritoku DK, Hulihan JF, Schwarzman LK, Kamin M, Olson WH. Effect of cotherapy reduction on tolerability of epilepsy add-on therapy: a randomized controlled trial. Ann Pharmacother. 2005;39:418–23.
48. Heo K, Lee BI, Yi SD, et al. Efficacy and safety of levetiracetam as adjunctive treatment of refractory partial seizures in a multicentre open-label single-arm trial in Korean patients. Seizure. 2007;16:402–9.
49. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15:106–15.
50. Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80:590–9.
51. Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62:258–61.
52. Kanner AM. Psychiatric comorbidities in new onset epilepsy: should they be always investigated? Seizure. 2017;49:79–82.
53. Kim SK, Park SP, Kwon OY. Impact of depression and anxiety on adverse event profiles in Korean people with epilepsy. Epilepsy Behav. 2015;46:185–91.
54. Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–82.
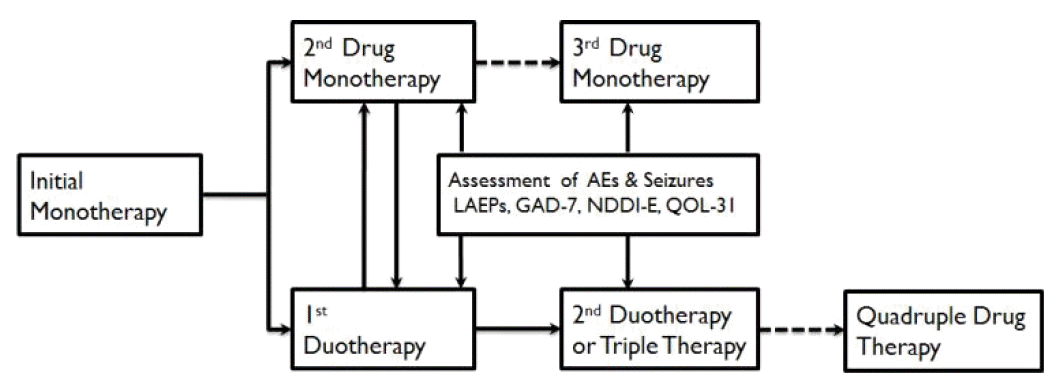
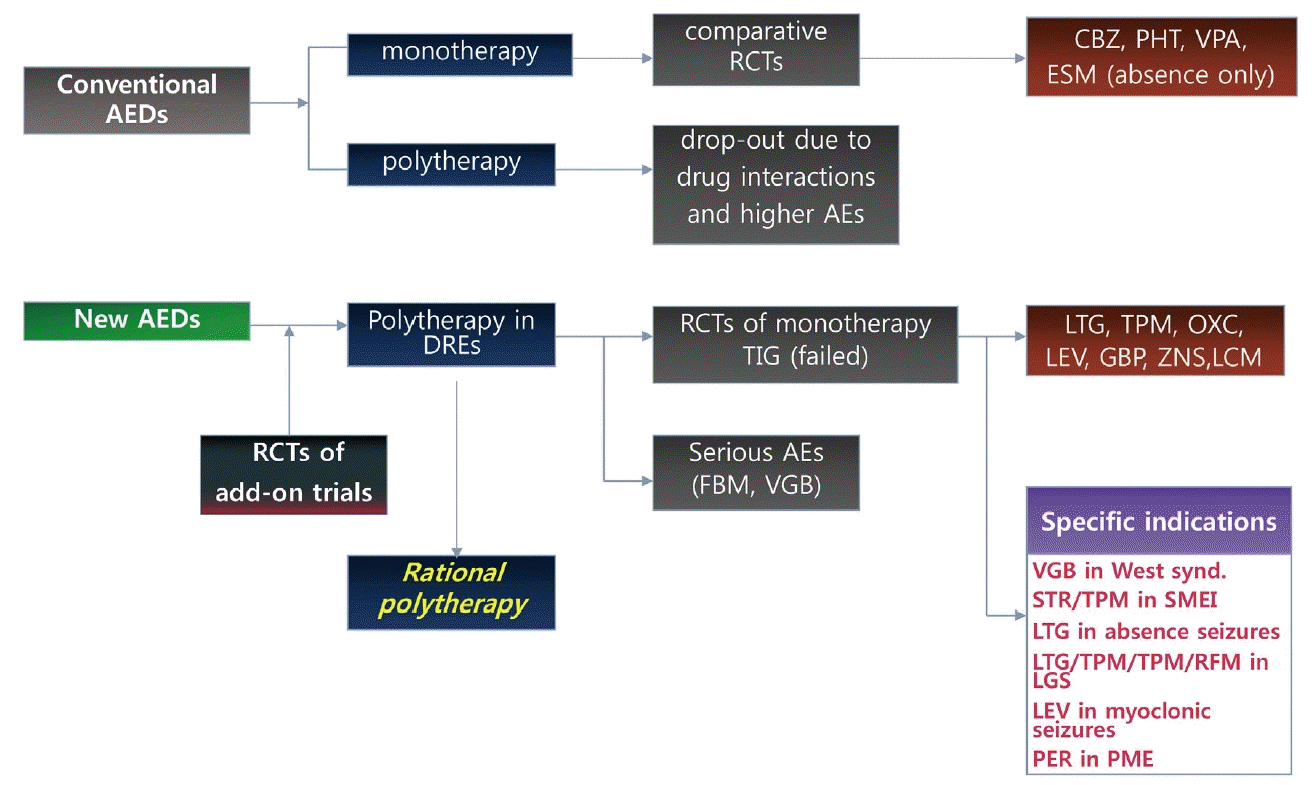
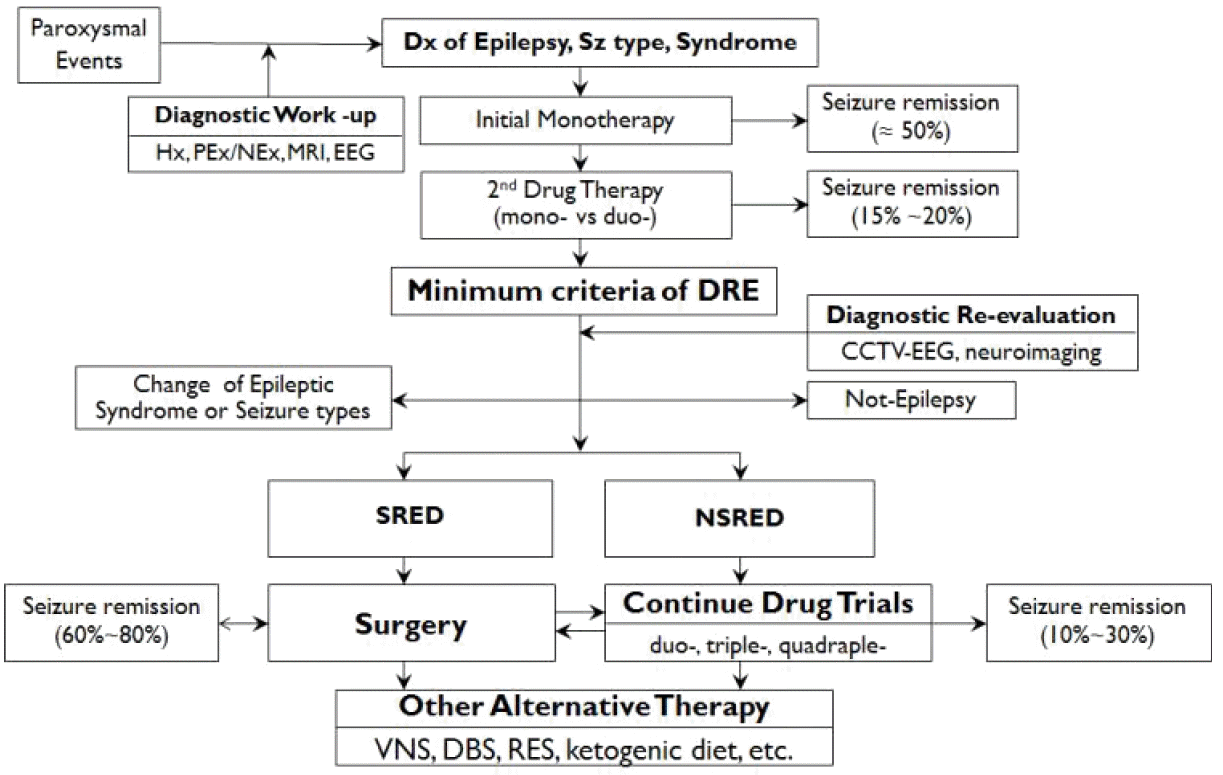
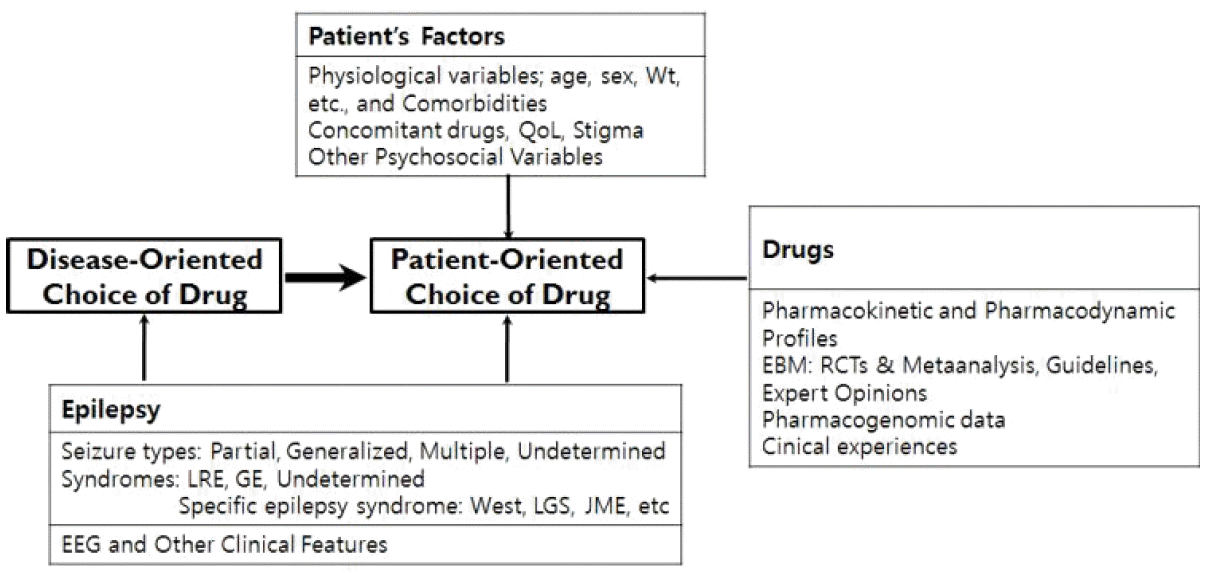
55. Lee BI, Park KM, Kim SE, Heo K. Clinical opinion: Earlier employment of polytherapy in sequential pharmacotherapy of epilepsy. Epilepsy Res. 2019;156:106165