AbstractBackground and Purpose:Cognitive dysfunction related to antiepileptic drugs (AEDs) is an important issue in the management of patients with epilepsy. The aim of the present study was to evaluate relative long-term effects of oxcarbazepine (OXC) on cognition in drug-naive patients with epilepsy.
Methods:Fifteen drug-naïve epilepsy patients were enrolled. Electroencephalogram (EEG) recordings and neuropsychological (NP) tests were performed before and after OXC monotherapy. The relative power of the discrete frequency bandwas obtained. In addition, interhemispheric and intrahemispheric spectral coherence was also calculated.
IntroductionCognitive dysfunction is frequently observed in epilepsy patients. As antiepileptic drugs (AEDs) are commonly taken for extended periods of time, the chronic use of AEDs may be a cause of cognitive dysfunction in this population.1–3 Thus, the impact of AED-related cognitive impairment on daily life is an important issue to consider in the treatment of epilepsy.
Oxcarbazepine (OXC) is a second generation AED that is chemically related to carbamazepine (CBZ) and approved as initial or add-on therapy for epilepsy patients. Neuropsychological (NP) tests show that, compared to traditional AEDs, OXC is not associated with significant cognitive side effects in both epilepsy patients and healthy subjects.2 While NP tests are a widely used method for detecting the effect of AEDs on cognitive function, there may be a significant test-retest variability and practice effect from repeated testing.4 Furthermore, NP tests cannot gather temporal information about cognitive processing by the brain.
Spectral analysis is based on changes in the electroencephalogram (EEG) spectrum, which reflects the functional state of the brain. Spectral analysis of electroencephalographic signals constitutes a helpful tool in the assessment of pharmacological effects of CNS drugs, such as AEDs, on the brain. It has proven to be a valuable technique for classifying psychopharmacological agents and assessing their pharmacodynamics. Therefore, it can serve as a simple and objective method to assess the relationship between AEDs and cognitive function.5–7 However, spectral EEG reflects only a general functional state of the brain including cognitive function. Given this drawback, NP tests are frequently necessary for proper interpretation of the spectral EEG findings.
To date, only a few studies examining OXC effects on spectral EEG have been reported in epilepsy patients.8,9 Clemens et al. reported OXC effects on spectral EEG in nine patients with epilepsy (mean age 18.8 years).8 They demonstrated that OXC had no significant effect on the spectral power in broad bands. Another study showed that OXC treatment was characterized by less delta, theta, and alpha power, and more beta power than carbamazepine treatment.9 However, as the study did not perform NP tests in conjunction with spectral EEG, the cognitive effects of OXC may be subject to misinterpretation.
In the present study, we investigated the effects of OXC on cognitive function using both NP tests and spectral power in an attempt to gather more useful information on cognitive impairment.5 We also adopted spectral coherence as a tool for identifying functional connectivity, given its unique ability to reveal synchronous interactions between two brain locations, which cannot be performed by the NP test or spectral EEG.10 Recently, spectral coherence approaches have been applied to observe functional connectivity in Alzheimer disease,11 epilepsy,12 alcoholism,13 and chronic fatigue syndrome.14
Material and MethodsSubject selection and OXC administrationInclusion criteria were as follows. 1) All subjects had a newly diagnosed epilepsy with at least 2 unprovoked partial or secondarily generalized seizures without previous exposure to AEDs. The diagnosis of epilepsy was made based on the revised classification of epilepsies and epileptic syndromes.15 2) The age of subjects limited to between 18 and 55 years. 3) Subjects had received EEG and NP tests twice, both before and after OXC treatment. Exclusion criteria included; 1) seizures evoked by metabolic abnormality, toxic exposure, active infection, or other treatable causes, 2) special seizure types such as absence, myoclonic, or atonic seizures, 3) use of acute anticonvulsant medications longer than one week, use of other central nervous system (CNS) acting drugs, 4) a diagnosis of mental retardation, progressive neurological disease or psychiatric disease, 5) pregnant or breat-feeding women or women who wanted to have baby within a year, 6) history of status epilepticus, and 7) abnormal laboratory findings including neutrophil count less than 1,800/mm3, platelet count less than 100,000/mm3, twice higher than upper normal range of aspartate aminotransferase (AST), alkaline aminotransferase (ALT), bilirubin, blood urea nitrogen (BUN), creatinine, sodium, and potassium levels. Fifteen patients met these criteria and were included in the final analysis.
Before OXC treatment, all subjects underwent a physical examination, routine blood tests including hematology and liver function tests, magnetic resonance imaging (MRI) of the brain, EEG, and NP tests. All tests were performed both at the study onset and 35 weeks (35.5± 6.39) post-drug initiation. OXC was given at 300 mg a day for the first two weeks, 600 mg a day for the next two weeks, then maintained with the same dosage. If seizures persisted after the initial target of dose 600 mg/day, the dosage was increased up to 1800 mg/dayuntil seizures were controlled. If a patient noted any intolerable side effects from OXC administration, the dosage was decreased by 300 mg a day for one or 2 weeks until symptoms subsided. A fixed doses of OXC were maintained for at least four weeks prior to conducting the second NP test and EEG study.
The frequency of seizures was assessed using a self-recorded seizure diaries brought in by subjects at subsequent outpatient clinic visits. Informed consent was obtained from all subjects in accordance with the institutional review board’s guidelines at each hospital participating in the study.
EEG recordingIn accordance with the international 10–20 system, EEGs were recorded using Ag/AgCl electrodes that were placed at Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2. Electrode impedance was kept below 5 kΩ, and the band-pass filter (0.5–70 Hz) was applied. The sampling rate was 200–400 Hz. The reference electrode was set to Pz electrode. Two electrooculography (EOG) channels (placed on the left and right outer canthi) were added to confirm eyeball movements and eliminate EOG artifacts. Subjects rested supine in a bed during EEG recordings and were asked to close their eyes for 10s and then open for another 10s. This procedure was repeated 10 times.
EEG data preprocessing and analysisEpoch selectionFor quantitative analysis of background EEG activity, an epilepsy specialist (JKY) selected epochs while blinded to the demographic data in our study. Epochs with artifacts or with epileptiform activities (spikes or sharp waves) were eliminated, while epochs with physiological alpha activity of maximum amplitude in occipital regions (O1 and O2 electrode) were selected. We collected 20 1.5 s-long epochs in each patient before and after OXC treatment.
Data preprocessingEEG data was preprocessed using EEGLAB version 10.0b16 managed in the MATLAB environment (version 7.10, The Mathworks, Natick, MA, USA). After down-sampling to 200 Hz, EEG data were re-referenced to the common average reference, and band pass filtered (0.5–50 Hz) for analysis.
Spectral powerTo identify spectral power, fast Fourier transform was applied to each of the selected epochs in the first step. Then, to improve the accuracy of spectral estimates all transformed epochs for each subject were averaged. Frequency band of 1 to 30 Hz with 0.4 Hz steps was used, and were then divided into seven discrete bands: Delta: 1–3 Hz, Theta: 3–7 Hz, Alpha 1: 7–10 Hz, Alpha 2: 10–13 Hz, Beta 1: 13–19 Hz, Beta 2: 19–24 Hz, and Beta 3: 24–30 Hz. In order to obtain relative powers, absolute powers of each frequency bin divided by sum of the overall spectral powers of each subject. Relative powers were log-transformed prior to statistical analysis. The electrodes were grouped into six scalp regions: LF (left frontal: Fp1, F7, F3), RF (right frontal: Fp2, F8, F4), LT (left temporal: T7, C3), RT (right temporal: T8, C4), LPO (left parieto-occipital: P3, P7, O1), and RPO (right parie-to-occipital: P4, P8, O2).
Spectral CoherenceTo calculate spectral coherence, magnitude square coherence (the mscohere function in signal processing toolbox of MATLAB) was used. Hanning windows were successively applied to 1.5s epochs of EEG with 50% overlap. Spectral coherence Cxy between two brain signals x(t) and y(t) was defined as
where Cxy was the cross-spectral density between x and y, and Gxx and Gyy were the autospectral densities of x and y, respectively. For spectral coherence calculation, spectral power ranged from 1 to 30 Hz and the interval was 0.4 Hz. Intrahemispheric spectral coherences were calculated for the eight electrode pairs (F3-C3, F4-C4, T3-T5, T4-T6, C3-P3, C4-P4, P3-O1 and P4-O2), and interhemispheric spectral coherences were computed for the six electrode pairs (F3-F4, T3-T4, T5-T6, C3-C4, P3-P4 and O1-O2) in each of the six discrete frequency bands.10
Neuropsychological assessmentsA series of NP tests were selected to investigate cognitive functions including attention, language, visuospatial abilities, motor skills, memory, and executive functions of the frontal lobes. The digit span test forward was administered to assess attention.17 Language was evaluated using the the short version of the Korean-Boston naming test (S-K-BNT) form A.18 Visuo-spatial function was evaluated using the Rey complex figure test (RCFT).19 Motor function was evaluated using the finger tapping test (FTT).20 To assess memory, we used two sub-tests: the Korean-California verbal learning test (K-CVLT)21 and the RCFT.19 To investigate the executive functions of the frontal lobe, we used five sub-tests: the controlled word association task (COWAT),22 the Korean-color word stroop test (K-CWST),23 the digit symbol test,24,25 the trail making test (TMT),26 and the Wisconsin card sorting test (WCST).27
Statistical analysisWilcoxon signed-rank tests were used to compare patients’s grand averages power spectra within each frequency bands, and NP test scores before and after the initiation of medication for each subject. Repeated measures analysis of variance (ANOVA) was used to analyze spectral powers of ROIs. The within-subject factors included treatment (two levels: baseline and follow-up) and electrode region (six levels: LF, RF, LT, RT, LPO, and RPO). Included among within-subject factors were cases of spectral coherence: electrode pair (eight levels for intrahemispheric spectral coherence, six levels for in-terhemispheric spectral coherence). The Greenhouse-Geisser correction was used to evaluate F ratios to control for type 1 errors in the repeated measures design. The significance level was set to 0.05. All statistical procedures were performed with SPSS (Version 12.0, Chicago, IL, USA).
ResultsSubjectsSubject demographic data and clinical characteristics are presented in Table 1. Seven of the fifteen subjects were men, and the mean age of the patients was 30.7±10.3 years (range: 18–55 years). The mean interval between EEGs was 248.5±46.3 days (range: 206–348 days). The mean daily dosage of OXC was 740±365.1 mg (range: 300–1,800 mg).
Quantitative EEG analysisSpectral analysisFor spectral power of each frequency band across all electrodes, the signed-rank test revealed no significant difference between baseline and follow-up EEGs (Fig. 1). Repeated measures ANOVA revealed no significant main effect of treatment on the spectral power of each frequency band at the ROIs. Spectral power was significantly different across regions (p<0.005). However, the interaction between treatment and regions was not significant (Fig. 2).
Spectral coherenceTo identify overall pattern of coherence at each frequency band across all electrode pair, we draw the line topographic map (Fig. 3). Threshold of coherence were selected values as represented by mean+1 SD of coherence at each frequency bands. Line topography of coherence revealed that there were no difference of patterns after treatment in all frequency bands.
A statistical summary of spectral coherence is presented in Table 2. For intrahemispheric coherence, repeated measures ANOVA revealed a significant main effect of treatment in all frequency bands. However, the interaction between treatment and electrode pair did not reach statistical significance. For interhemispheric coherence, statistical results were the same as intrahemispheric coherence.
Neuropsychological testsStatistical results of the NP tests are listed in Table 3. Significant differences were observed in the RCFT copy time (Z=−1.079, p=0.031), K-CVLT 1–5 trial total (Z=−1.929, p=0.054), digit symbol test (Z=−2.639, p=0.008), and TMT-B (Z=−1.988, p=0.047). The time to copy the Rey complex figure and to complete TMT-B was significantly decreased, while scores for the K-CVLT 1–5 trial total and digit symbol test were significantly increased. Significant differences were not noted in the other tests.
DiscussionIn the present study, we investigated the effect of OXC monotherapy on background EEG spectral power, spectral coherence, and found that OXC was not associated with a significant change in spectral power or coherence; however, some cognitive functions improved. NP tests demonstrated improved performance in the copy trial of the RCFT (visuospatial function), K-CVLT (memory), digit symbol test, and TMT-B (executive function of frontal lobes). These findings are consistent with previous studies demonstrating OXC’s positive effects on a wide range of cognitive functions such as visuo-spatial organization,28,29 memory,30 focused attention, manual writing speed,31 and mental processing speed.29
No significant changes in frequency band powers were observed with OXC treatment, which is congruent with previous study.8 Although the effects of OXC on spectral EEG have been reported, the functional connection between brain regions remains unclear. Spectral coherence may be interpreted as the frequency-indexed correlation coefficient estimating the linear relationship between two time series. Thus, coherence means synchrony or coupling of two regions in a given frequency band.32 Neither intrahemispheric nor interhemispheric coherence changed significantly for all electrode pairs, indicating that OXC did not have a significant effect on the functional connectivity of EEG in our study.
Spectral coherence has been the main method used to assess the degree of functional connectivity between brain areas and associations between different functional states, however, the main limitation of this approach is that it investigates only a linear statistical link between EEG signals. Therefore, one cannot rule out the possibility of changes in nonlinear functional connectivity between brain regions following OXC treatment.33,34
AEDs can be categorized into two groups according to their effect on both spectral EEG and cognitive function (Table 4). Most older AEDs, including phenytoin35–37 and carbamazepine,8,9,37–41 as well as newer AEDs such as topiramate42–46 and gabapentine,40,45 tend to increase delta and theta power and decrease higher frequency bands. This first category of AEDs negatively affects cognitive function in patients with epilepsy. Increased EEG slowing is well correlated with cognitive decline in demented patients.47 In the case of carbamazepine and phenytoin, reported slowing of EEG background rhythm has been positively correlated with negative neuropsychological results as measured by cognitive testing.37,38,40,48
On the other hand, lamotrigine,8,43,46 levetiracetam5,39,49 and valproate8,50 are reported to reduce the power of slow frequencies and/or increase that of fast frequencies, thereby improving some cognitive function in diverse NP tests.2,51,52 In subjects treated with levetiracetam, the alpha and beta bands were positively correlated with improved neuropsychological results.5,53
Unlike AEDs of two categories above, OXC had no significant effect on spectral EEG, despite some cognitive functions were improved with OXC treatment. Thus, OXC seems to be an unique pharamacological effect on the brain in patients with epilepsy. However, as the number of patients was relatively small in the present study, larger sample of the study will be required to corroborate our study.
Brain activity during the resting state may not be captured on EEG compared with a cognitively functioning brain state that demonstrates changes in attention, inhibition, memory, and so forth. Moreover, it has been reported that there is no direct or causal relationship between EEG background measures and cognitive functions.7,49 This finding might suggest discrepancies between resting state spectral EEG and NP tests in our study. Accordingly, future studies should consider event-related potential study in addition to resting state EEG in order to better identify the effects of AEDs on cognitive function. In conclusion, our study supports the notion that OXC has no significant cognitive side effect in patients with epilepsy.
AcknowledgmentsThis study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A090794) to KYJ, and by the Ewha Global Top 5 Grant 2011 of Ewha Womans University and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R01-2011-0015788) to HWL and (No. A110097) to SBH.
Figure 1Total average of relative power for all patients and all channels within each frequency band. There was not a significant change in relative frequency band power between conditions. RD, Relative Delta; RT, Relative Theta; RA1, Relative Alpha1; RA2, Relative Alpha2; RB1, Relative Beta1; RB2, Relative Beta2; RB3, Relative Beta3. 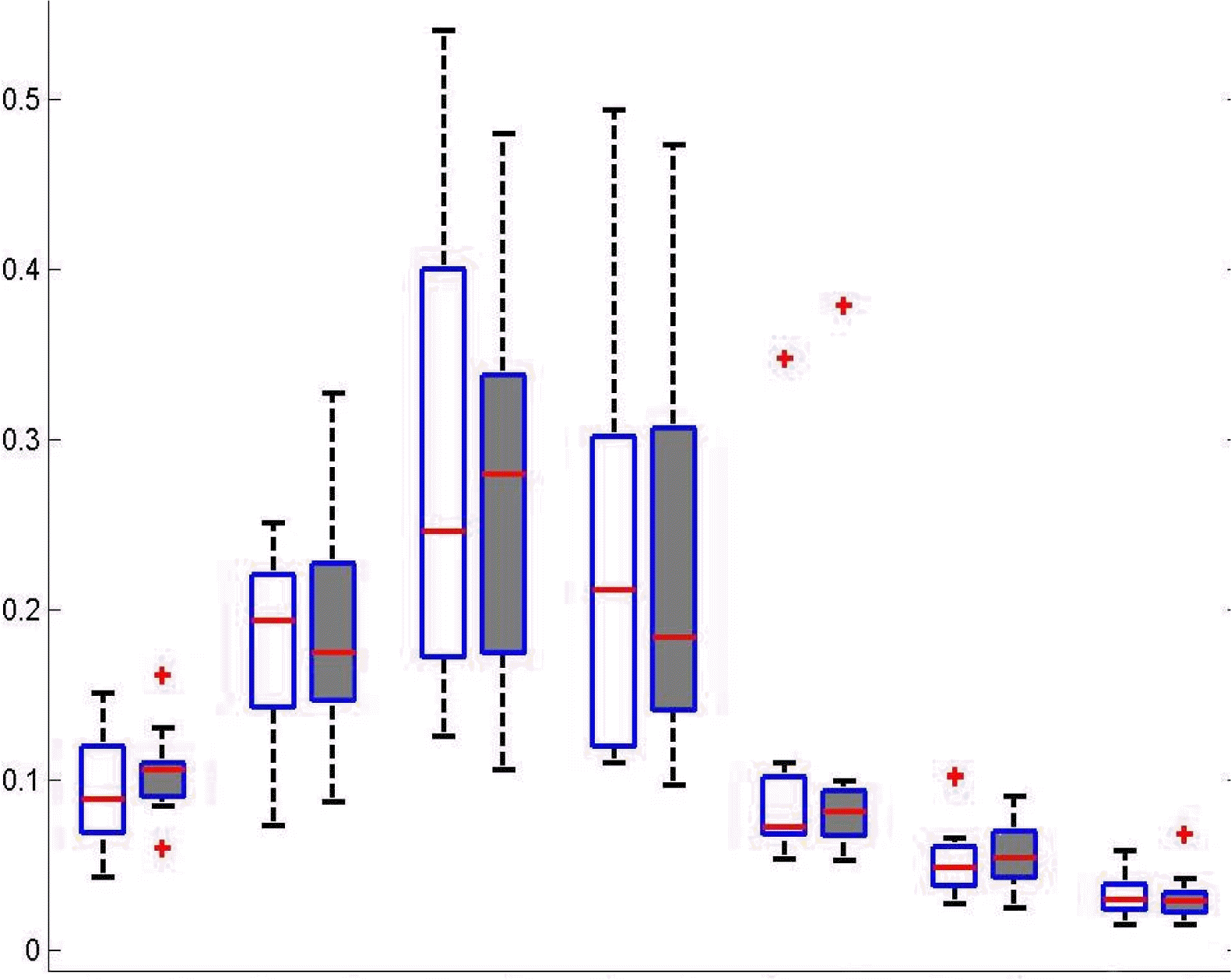
Figure 2Relative power of each of seven frequency bands at six regions of interest. LF, left frontal; RF, right frontal; LT, left temporal; RT, right temporal; LPO, left parieto-occipital; RPO, right parieto-occipital; RD, Relative Delta; RT, Relative Theta; RA1, Relative Alpha1; RA2, Relative Alpha2; RB1, Relative Beta1; RB2, Relative Beta2; RB3, Relative Beta3. 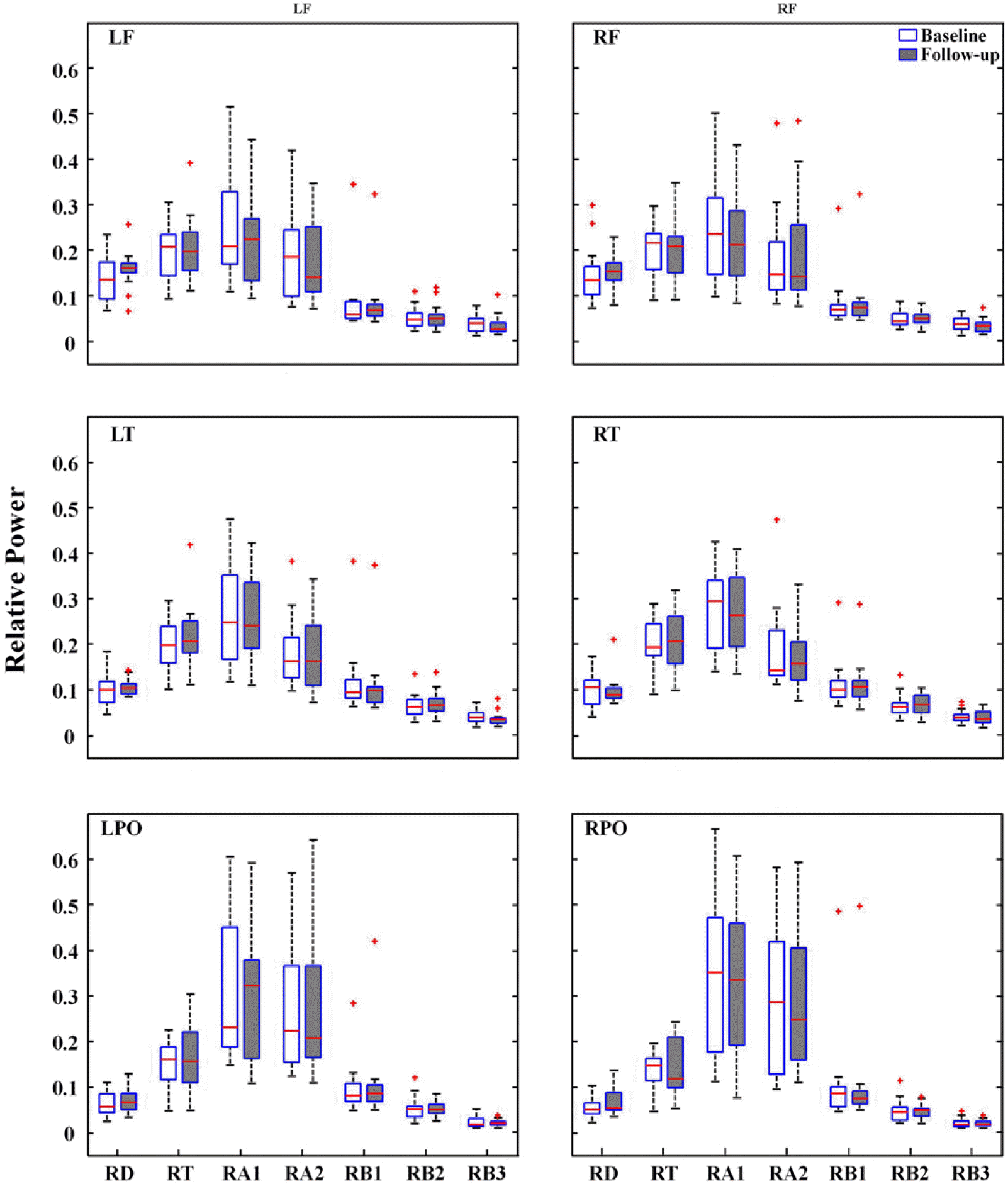
Figure 3The overall pattern of line topographic map of coherence before and after treatment with oxcarbazepine. The Line of topographic maps indicate that coherence value over mean value with added to one standard deviation of each frequency band. Threshold value are written under the topographic map. The thickness of line indicates the relative strength of coherence. 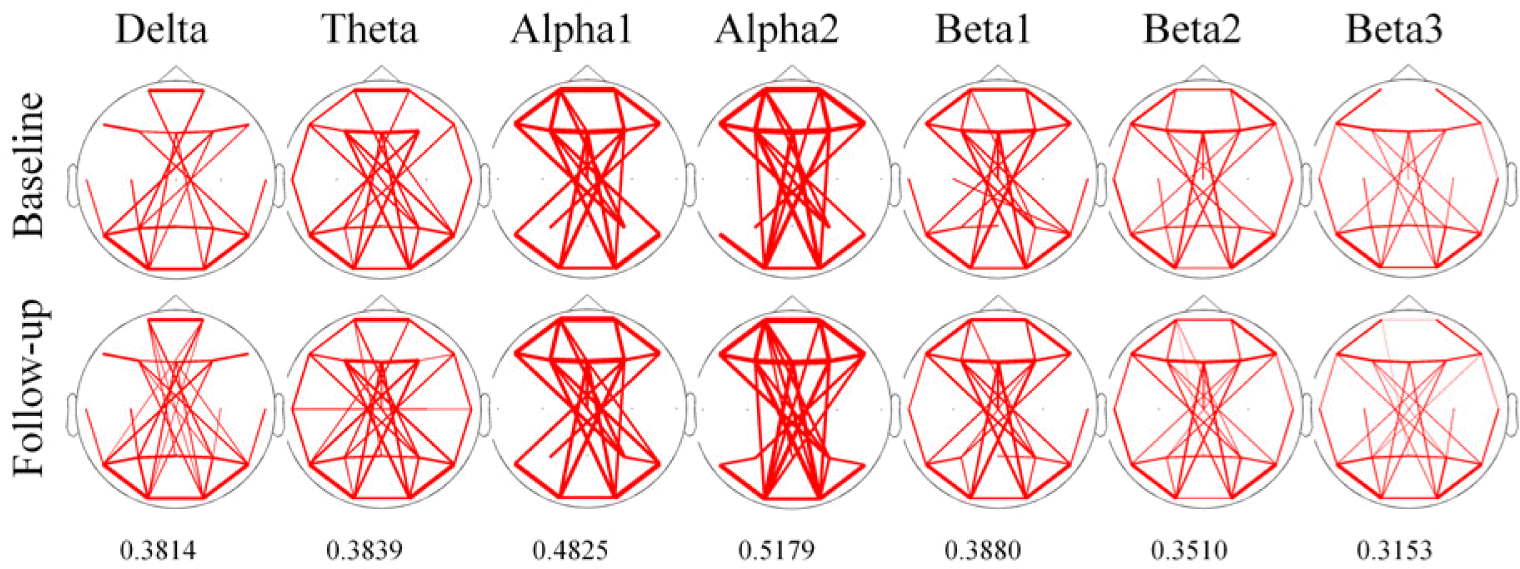
Table 1Clinical characteristics of the study population Table 2Repeated measures analysis of variance (ANOVA) of spectral coherence at discrete frequency bands of all electrode pairs Table 3Result for neuropsychological examinations
Table 4Effect of AEDs on EEG spectral power and cognition
References1. Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Res. 2006. 68:63–7.
3. Thompson PJ, Trimble MR. Anticonvulsant serum levels: relationship to impairments of cognitive functioning. J Neurol Neurosurg Psychiatry. 1983. 46:227–33.
4. Salinsky MC, Storzbach D, Dodrill CB, Binder LM. Test-retest bias, reliability, and regression equations for neuropsychological measures repeated over a 12–16-week period. J Int Neuropsychol Soc. 2001. 7:597–605.
5. Cho JR, Koo DL, Joo EY, et al. Effect of levetiracetam monotherapy on background EEG activity and cognition in drug-naïve epilepsy patients. Clin Neurophysiol. 2012. 123:883–91.
6. Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002. 43:482–90.
7. Salinsky MC, Oken BS, Storzbach D, Dodrill CB. Assessment of CNS effects of antiepileptic drugs by using quantitative EEG measures. Epilepsia. 2003. 44:1042–50.
8. Clemens B, Menes A, Piros P, et al. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006. 70:190–9.
9. Clemens B, Ménes A, Nagy Z. Objective assessment of neurotoxicity while shifting from carbamazepine to oxcarbazepine. Acta Neurol Scand. 2004. 109:324–9.
10. Adler G, Brassen S, Jajcevic A. EEG coherence in Alzheimer’s dementia. J Neural Transm. 2003. 110:1051–8.
11. Fonseca LC, Tedrus GM, Prandi LR, Almeida AM FD. Alzheimer’s disease: relationship between cognitive aspects and power and coherence EEG measures. Arq Neuropsiquiatr. 2011. 69:875–81.
12. Moller DW, Chiu AWL. Noise-assisted intrinsic mode function coherence in seizure anticipation. Conf Proc IEEE Eng Med Biol Soc. 2011. 2011:8287–90.
13. Tcheslavski GV, Gonen FF. Alcoholism-related alterations in spectrum, coherence, and phase synchrony of topical electroencephalogram. Comput Biol Med. 2012. 42:394–401.
14. Duffy FH, McAnulty GB, McCreary MC, Cuchural GJ, Komaroff AL. EEG spectral coherence data distinguish chronic fatigue syndrome patients from healthy controls and depressed patients--a case control study. BMC neurol. 2011. 11:82
15. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989. 30:389–99.
16. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004. 134:9–21.
17. Wechlser D. Wechsler memory scale-Revised manual. San Antonio, TX: The Psychological Corporation; 1987.
18. Kim H, Na D. Parallel Short Forms for the Korean-Boston Naming Test (K-BNT). Seoul: Hakjisa; 2000.
19. Meyers JE, Meyers KR. Rey complex figure test and recognition trial. Psychological assessment resources Inc; 1995.
20. Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery theory and interpretation. Neuropsychology Press; 1985.
21. Kim JK, Kang YW. Korean-California verbal learning test. Seoul: Special Education; 1997.
22. Kang Y, Na D. Seoul neuropsychological screening battery. Seoul: Human Brain Research & Consulting; 2003.
23. Lee J, Kang Y, Na D. Efficiencies of the Stroop interference indexes in healthy older adults and dementia patients. Korean J Clin Psychol. 2000. 19:807–18.
24. Stephens R, Kaufman A. The role of long-term memory in digit-symbol test performance in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009. 16:219–40.
25. Wechsler D. Wechsler Adult Intelligence Scale - revised manual. New York: The Psychological Corporation; 1981.
26. Reitan RM. Trail making test: manual for administration and scoring. Tucson AZ: Neuropsychology Press; 1992.
27. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin card sorting test manual revised and expanded. Lutz, FL: Psychological Assessment Resources, Inc; 1993.
28. Tzitiridou M, Panou T, Ramantani G, Kambas A, Spyroglou K, Panteliadis C. Oxcarbazepine monotherapy in benign childhood epilepsy with centrotemporal spikes: a clinical and cognitive evaluation. Epilepsy Behav. 2005. 7:458–67.
29. Donati F, Gobbi G, Campistol J, et al. The cognitive effects of oxcarbazepine versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizure. 2007. 16:670–79.
30. Kim SY, Lee HW, Jung DK, Suh CK, Park SP. Cognitive Effects of Low-dose Topiramate Compared with Oxcarbazepine in Epilepsy Patients. J Clin Neurol. 2006. 2:126–33.
31. Curran HV, Java R. Memory and psychomotor effects of oxcarbazepine in healthy human volunteers. Eur J Clin Pharmacol. 1993. 44:529–33.
32. Bullock TH, McClunea MC. Lateral coherence of the electrocorticogram: a new measure of brain synchrony. Electroencephalogr Clin Neurophysiol. 1989. 73:479–98.
33. Sherman D, Zhang N, Garg S, et al. Detection of nonlinear interactions of EEG alpha waves in the brain by a new coherence measure and its application to epilepsy and anti-epileptic drug therapy. Int J Neural Syst. 2011. 21:115–26.
34. Alonso JF, Mañanas MA, Romero S, Hoyer D, Riba J, Barbanoj MJ. Drug effect on EEG connectivity assessed by linear and nonlinear couplings. Hum Brain Mapp. 2010. 31:487–97.
35. Salinsky MC, Spencer DC, Oken BS, Storzbach D. Effects of oxcarbazepine and phenytoin on the EEG and cognition in healthy volunteers. Epilepsy Behav. 2004. 5:894–902.
36. Herkes GK, Lagerlund TD, Sharbrough FW EM. Effects of antiepileptic drug treatment on the background frequency of EEGs in epileptic patients. J Clin Neurophysiol. 1993. 10:210–6.
37. Meador KJ, Loring DW, Abney OL, Allen ME, Moore EE, Zamrini EY KD. Effects of carbamazepine and phenytoin on EEG and memory in healthy adults. Epilepsia. 1993. 34:153–7.
38. Frost JD, Hrachovy RA, Glaze DG, Rettig GM. Alpha rhythm slowing during initiation of carbamazepine therapy: implications for future cognitive performance. J Clin Neurophysiol. 1995. 12:57–63.
39. Mecarelli O, Vicenzini E, Pulitano P, et al. Clinical, cognitive, and neurophysiologic correlates of short-term treatment with carbamazepine, oxcarbazepine, and levetiracetam in healthy volunteers. Ann Pharmacother. 2004. 38:1816–22.
40. Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002. 43:482–90.
41. Besser R, Hornung K, Theisohn M, Rothacher G, Krämer G. EEG changes in patients during the introduction of carbamazepine. Electroencephalogr Clin Neurophysiol. 1992. 83:19–23.
42. Mecarelli O, Piacenti A, Pulitano P, et al. Clinical and electroencephalographic effects of topiramate in patients with epilepsy and healthy volunteers. Clin Neuropharmacol. 2001. 24:284–9.
43. Neufeld MY, Kogan E, Chistik V, Korczyn AD. Comparison of the effects of vigabatrin, lamotrigine, and topiramate on quantitative EEGs in patients with epilepsy. Clin Neuropharmacol. 1999. 22:80–6.
44. Placidi F, Tombini M, Romigi A, et al. Topiramate: effect on EEG in-terictal abnormalities and background activity in patients affected by focal epilepsy. Epilepsy Res. 2004. 58:43–52.
45. Salinsky M, Storzbach D, Oken B, Spencer D. Topiramate effects on the EEG and alertness in healthy volunteers: a different profile of an-tiepileptic drug neurotoxicity. Epilepsy Behav. 2007. 10:463–9.
46. Smith ME, Gevins A, Mcevoy LK, Meador KJ, Ray PG, Gilliam F. Distinct Cognitive Neurophysiologic Profiles for Lamotrigine and Topiramate. Epilepsia. 2006. 47:695–703.
47. Roh JH, Park MH, Ko D, et al. Region and frequency specific changes of spectral power in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2011. 122:2169–76.
48. Herkes GK, Lagerlund TD, Sharbrough FW, Eadie MJ. Effects of Antiepileptic Drug Treatment on the Background Frequency of EEGs in Epileptic Patients. J Clin Neurophysiol. 1993. 10:210–6.
49. Veauthier J, Haettig H, Meencke HJ. Impact of levetiracetam add-on therapy on different EEG occipital frequencies in epileptic patients. Seizure. 2009. 18:392–5.
50. Béla C, Mónika B, Márton T, István K. Valproate selectively reduces EEG activity in anterior parts of the cortex in patients with idiopathic generalized epilepsy. A low resolution electromagnetic tomography (LORETA) study. Epilepsy Res. 2007. 75:186–91.
51. Meador KJ, Baker GA. Behavioral and Cognitive Effects of Lamotrigine. J Child Neurol. 1997. 12:44–7.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||