AbstractBackground and PurposeImplantation of deep brain stimulation (DBS) electrodes in the anterior nucleus of the thalamus (ANT) or the centromedian nucleus (CM), for the treatment of refractory epilepsy, is technically demanding. To enhance the accuracy of electrode placement within the ANT and CM, we analyzed our experience with electrode revision surgery in ANT and CM DBS and investigated the cause of misplacement and verifying methods for accurate placement.
MethodsA retrospective analysis of the medical records of 23 patients who underwent DBS for refractory epilepsy during the period from 2013 to 2016 was performed.
ResultsMisplacement of the electrode occurred in 1 (25%) of 4 ANT DBS and 2 (14.3%) of 14 patients with CM DBS performed in our institute, and revision surgery was performed in three patients. During this period, we performed three revision surgeries for misplaced electrodes in ANT DBS that were performed at another hospital. Therefore, we performed six revision surgeries (four in ANT, two in CM) for mistargeted DBS electrodes for thalamic DBS. Transventricular lead placement and an anatomical targeting of the ANT was the cause of misplacement in the ANT and intraoperative brain shift was found to be the cause in the CM. For verification of the location of lead placement, magnetic resonance imaging (MRI) was superior to computed tomography and electroencephalography (EEG).
ConclusionsTo reduce the rate of electrode misplacement for refractory epilepsy, image-based targeting of the ANT according to individual anatomical variation, and efforts to minimize intraoperative brain shift are essential. To verify the location of the electrode, MRI examination is mandatory in DBS for refractory epilepsy.
IntroductionDeep brain stimulation (DBS) of the thalamus is an emerging treatment for patients with medically refractory epilepsy who are not suitable candidates for resective surgery.1,2 Several clinical studies have tested the efficacy of stimulation of different brain structures, including the anterior thalamic nucleus (ANT), centromedian nucleus (CM), subthalamic nucleus, medial temporal structures, nucleus accumbens, and other cortical structures.1 Among several targets of DBS for refractory epilepsy, ANT and CM stimulation have been reported to be effective for refractory epilepsies with partial-onset and generalized seizures.2–10
Because the ANT is located in the medial, superior corner of the thalamus facing the lateral and third ventricles, implantation of the DBS electrode in the ANT is performed through a transventricular approach using the lateral ventricle.4,10 However, the transventricular route for lead implantation is associated with targeting errors. Planning an electrode trajectory that avoids the ventricle has been suggested as a simple precaution that significantly improves the accuracy of anatomical targeting during DBS.11 Considering that replacement of misplaced DBS electrodes located outside the ANT was required in 8.2% of the 110 patients enrolled in the SANTE trial,10 accurate placement of the DBS electrode within the ANT can be difficult to achieve.4,10 Indeed, thalamic size and shape vary significantly across patients, with changes specific to the ANT occurring with age and in the setting of chronic epilepsy.12–16 These variations in thalamic shape and volume necessitate the direct targeting of the ANT. We reviewed our cases of mistargeting and analyzed the causes of technical failure. We suggest technical considerations in the planning and conducting of electrode implantation into the thalamus in order to minimize mistargeting and to enhance accuracy of electrode implantation, and subsequently to increase the success rate of thalamic DBS for refractory epilepsy.
MethodsThe medical records of 23 patients who underwent DBS for refractory epilepsy from June 2013 to March 2016 were reviewed. Seven underwent DBS in the ANT and 15 had CM DBS procedures. One patient underwent bilateral DBS of the hippocampus. The selection of patients, presurgical evaluation, target determination, and DBS procedures have been previously reported.9,12,17
Surgical procedures and anatomical target localizationA Leksell model G stereotactic head frame (Elekta Instruments, Atlanta, GA, USA) was applied and a stereotactic magnetic resonance imaging (MRI) scan (1.5T) was performed using an Archieva® apparatus (Philips, Best, Netherlands). Axial T2-weighted images and volumetric T1 three-dimensional (3D) sequences were acquired and transferred to a Framelink® ver. 4.1 planning software system (Medtronic, Minneapolis, MN, USA) to determine the coordinates of the anterior (AC) and posterior commissure (PC), and reformatted for trajectory planning. Due to a high degree of individual anatomical variation in the location of the ANT,5,14–18 a direct MRI-guided target localization and trajectory planning was performed to include the ANT and medi-odorsal (MD) nuclei for ANT DBS. Anatomical target coordinates of the CM nucleus were 10 mm lateral from midline, 1 mm anterior to the PC, and 1 mm above the intercommissural line (ICL), consistent with those suggested by Velasco et al.19 Implantation of model 3389 bilateral quadripolar electrodes (Medtronic) was performed under general anesthesia, with the exception of the first two patients. For these patients, the operation was performed under local anesthesia with microelectrode recording (MER) and monitoring intraoperative scalp EEG (driving) response to macroelectrode stimulation. However, considering the vague prognostic values of MER and acute intra-operative EEG driving response, and to reduce patient discomfort, subsequent operations were performed under general anesthesia.9,12
Electrical stimulation with a model 3628 dual screen external stimulator (Medtronic) and simultaneous EEG recording (Grass Technology, West Warwick, RI, USA) were conducted on the day following electrode implantation. Bipolar high-intensity, low-frequency stimuli (5 Hz, 5–10 V, 130 μs) were delivered through all possible combinations of pairs among the four contacts.9,12 The duration of each stimulation session was 3 minutes, followed by an off-stimulation period of at least 2 minutes. Cerebral synchronizing and driving response (DR) was pursued during bipolar stimulation. Subsequent, unblinded trial stimulation under continuous EEG monitoring, through the pairs of contacts showing a strong DR, was conducted for 2–3 days. The implantation of pulse generators (Soletra®, model 7426; Medtronic) was performed 3 to 7 days after electrode implantation, under general anesthesia, through a transaxillary subpectoral route.20
Localization and verification of electrodesImages of immediate postoperative computed tomography (CT, 1-mm-thick axial slices) were transferred and merged to preoperative volumetric T1-weighted magnetic resonance (MR) images to verify the location of the electrodes.9,12 The location of the quadripolar electrodes was assessed using a fusion of the postoperative CT images on the preoperative volumetric T1 MR images in the Framelink® software (Medtronic). The center of the contacts used as cathodes was identified with adjustment of contrast and brightness, and lead location was then measured as a variable in three dimensions with respect to the PC.9,12 In addition, the laterality from the wall of the third ventricle, as measured on the axial plane corresponding to the center of the active contact, was measured, because we often encountered a large third ventricle in refractory epilepsy patients with encephalomalacia and resultant difficulty in determination of the laterality of target coordinates. Lead locations were plotted with respect to a drawing adapted from a stereotactic atlas of the human thalamus.21 Targeting of the electrode was defined as successful when at least two electrode contacts were identified within the ANT or CM in postoperative CT/preoperative MRI fusion images.
ResultsMistargeting of the electrode occurred in 3 of 19 patients (15.8%), that is, in 7.9% of a total of 38 electrodes. It occurred in one (25%) of the four ANT DBS and two (14.3%) of the 14 patients with CM DBS performed in our institute. Relocation of the electrode was performed in three patients. During the study period, we performed three cases of revision surgery for misplaced electrodes in ANT DBS that were performed at another hospital. Therefore, we performed six revision surgeries (four in the ANT, two in the CM) for mistargeted DBS electrodes for thalamic DBS. The causes of mistargeting by imaging study are summarized in Table 1.
In three patients who had revision surgery for mistargeted electrodes in our hospital, suboptimal placement was found during the immediate postoperative EEG and imaging studies. In the patient with a misplaced ANT electrode (patient #1), it occurred on the right side (Fig. 1), and bilateral electrodes were found to be misplaced in two patients with CM DBS procedures (Fig. 2). The driving response of the EEG was not elicited in all five misplaced electrodes in both the ANT and CM nuclei. A computed tomographic (CT) scan, even with thin imaging slices (1 mm thickness), could not identify the misplaced ATN electrode due to metallic artifact caused by electrode contacts in patient #1. However, postoperative MRI was diagnostic for the misplacement in all five electrodes, including a suboptimally placed ANT electrode.
In three patients whose misplaced ANT electrodes were implanted in another institution, the misplacement was found later during follow-up in our institute; for these cases, MRI rather than CT scan was quite effective in the assessment of the actual electrode location. In patient #6, the CT scan was not informative for detecting misplacement because of a metallic artifact (Fig. 3). Indeed, the driving response of the EEG and a CT scan could not be systematically performed in these patients. They performed an MRI as an evaluation of decreased anti-epileptic efficacy of their chronic ANT stimulation. Unfortunately, we did not have access to their immediate postoperative imaging, performed five to seven years previously. As a diagnostic means to assess actual electrode location and misplacement, MRI was quite effective in the evaluation of all six patients. All four of the misplaced ANT electrodes were discovered to be within the third ventricle.
The cause of mistargeting was analyzed for each case. The causes of mistargeting during lead placement in the ANT was determined to be transventricular lead placement (the three patients with DBS performed at the other institute) and an anatomical targeting of the ANT nucleus (one patient in our institute). Mistargeting of the CM consisted of an intraoperative brain shift (two patients). A possible factor influencing the accuracy of lead placement was the individual variation in each patient’s brain anatomy. Indeed, there were two cases of schizencephaly with asymmetrical brain involving the hemisphere and the thalamus, while ventriculomegaly was found in the other two patients.
DiscussionTargeting of ANT and CMConsidering that replacement of misplaced DBS electrodes located outside the ANT was required in 8.2% of 110 patients enrolled in the SANTE trial,10 accurate placement of the DBS electrode within the ANT may be difficult to achieve.12 Although we experienced only four patients with primary ANT DBS, misplacement occurred in one out of five electrodes intended for placement within the ANT. Currently, there are no reports on the analysis of misplacement of DBS electrodes within the ANT. Therefore, it is difficult to draw general conclusions about the technical difficulty of electrode implantation into the ANT.12 Indeed, we experienced some difficulty during initial electrode implantation in the ANT. In 2013, we were unable to obtain detailed information regarding the target coordinates of the ANT because target coordinates reported in previous literature differed from center to center, and the descriptions were often vague.22 In our review, the reported anatomical coordinates of the X, Y, Z axes for ANT among 16 reports varied approximately 3 mm.22 As seen in our initial misplacement that occurred in patient #1, we experienced a trial and error process due to an initial electrode implantation performed according to anatomical targeting. Since that time, we changed our strategy for lead implantation from anatomical targeting to image-based targeting.
Limitations of indirect anatomical targeting and the importance of image-guided targeting in the ANT have recently been emphasized.5,15,16,18 Direct targeting based on individual visualization of the ANT with improved MRI imaging techniques has largely replaced indirect anatomical targeting using the AC-PC coordinates system.16 Significant variation in the size and shape of the thalamus has been reported. Thalamic volume decreases with age and this decrease is more prominent in the medial and anterior portion of the thalamus, where the ANT is located.14–16 Thalamic atrophy has been demonstrated in patients with epilepsy, and this atrophy is more pronounced in the side ipsilateral to seizure onset, in patients with temporal lobe atrophy.14–16 Such variability and ambiguity of the anterior thalamus pose significant concerns regarding electrode position and have potential implications on clinical outcomes.16
In order to improve the accuracy of the image-based targeting, better delineation of the ANT in the stereotactic imaging is an essential aspect of electrode implantation. Buetjen et al.17 suggested 3T MRI-based targeting of the anteroventral (AV) subnucleus of the ANT with T1 magnetization-prepared rapid aquisition of gradient echo (MPRAGE) imaging of the thalamus, which enables visualization of the white matter laminae around the ANR. Möttönen et al.16 reported that the ANT was more clearly delineated with 3T MRI short tau inversion recovery images, demonstrating white matter structures, such as the internal and external medullary laminae, surrounding the ANT. They suggested that direct targeting in the ANT was superior to indirect targeting with MER due to extensive individual variation in the location of the ANT.16 This anatomical variation of thalamic anatomy across individuals was again suggested by a recent study that analyzed the location of effective stimulation electrodes with MRI in six patients who had been included in the SANTE trial.15 We have summarized recent reports describing enhanced identification of the ANT for MRI imaging-guided targeting (shown in Table 2).
Although the exact location of the stimulation target is not well defined, several studies have suggested that the electrode contacts in successful treatments were located more anterior and superior within the ANT.5,15,16 Indeed, the ANT consists of 3 subnuclei: the AV, anteromedial (AM), and anterodorsal (AD) subnuclei.23 The AV sub-nucleus is the largest of the three subnuclei and has extensive connections with the hippocampus, subiculum, and cingulate cortex, and conveys theta rhythm activity that promotes synaptic plasticity within the hippocampal circuit. The AM subnucleus has reciprocal connections with the anterior cingulate and orbitomedial prefrontal cortex23 and is more involved in cognitive, emotional, and executive functions.23 The AD subnucleus is part of a network involving the lateral mammillary nucleus and is associated with spatial navigation and memory.23 Therefore, superior and anterior stimulation within the ANT would affect primarily the AV subnucleus, which has the most extensive connection with temporal and limbic circuits.23 In concordance with this anatomical consideration, Lehtimäki et al.5 suggested that anterior and superior stimulation within the ANT, affecting primarily the AV subnucleus, may result in better seizure control. These anatomical and clinical results suggest that accurate electrode placement within the ANT, specifically involving the AV subnucleus, and the direct targeting of the ANT based on individual anatomical variation, are important considerations for successful DBS procedures.5,16
Verification of accurate electrode placementAccurate placement of leads within the thalamic subnucleus can be assessed with a clinical effect, postoperative EEG driving response, and radiological examinations. Although immediate reduction of seizure frequency following lead implantation has been observed in some cases, it is difficult to determine the accurate placement of leads within several days after operation. Driving response (DR) or a recruiting response is a rhythmic cortical EEG synchronization following low-frequency stimulation of the thalamus12,24,25 and cortical EEG synchronization following thalamic stimulation has been suggested to be a useful marker not only for positioning within the centromedian nucleus but also for an estimation of the clinical efficacy of the thalamic CM DBS.26 However, the spatial specificity of the DR within the thalamus has been questioned because a DR can be elicited from nuclei other than the ANT, including the CM, MD, and ANT in humans.10,24–26 Therefore, the presence of a DR was not interpreted as evidence that the electrodes were placed specifically in the ANT or CM, but rather that they were placed in thalamic tissue.6,12 Furthermore, the DR was observed by external stimulation with a low-frequency, high intensity (7–9 V) stimulus to a misplaced electrode in the third ventricle, closely facing ANT.12 Therefore, interpretation of the DR should be done carefully. It was suggested that if the DR could not be observed, it should be regarded as a misplacement, and even if the DR is observed, it should not be regarded as a necessary and sufficient condition suggesting that the DBS electrode is within thalamic tissue.12 Son et al.12 suggested that the diagnostic importance of DR in the verification of electrode placement within thalamic nuclei is limited, and a postoperative, sophisticated imaging study is needed in all cases of ANT DBS in order to improve long-term outcomes in refractory epilepsy.
It would seem that radiological examination is essential in the verification of the position of the electrode within the target nucleus immediately after lead implantation. Among the radiologic examination modalities, CT scan is an easily applicable imaging modality and can be performed within minutes. However, diagnostic accuracy in the determination of the lead location within the ANT is limited by the metallic artifacts generated by two closely approximated, bilateral electrodes, which makes it difficult to identify a misplaced electrode in the third ventricle (Fig. 1A). In our study, it was difficult to determine electrode misplacement within the third ventricle with the CT scan. However, misplacement was easily identified by MRI examination in all cases in which suspicion of misplacement existed (Table 1). Therefore, to verify the exact location of the electrode in the ANT or to confirm misplacement within the third ventricle, MRI appears to be an essential imaging tool in DBS for refractory epilepsy.
To enhance the more accurate placement of electrodes in thalamic subnucleus. for the treatment of refractory epilepsy, imaging-guided targeting and efforts to reduce intraoperative brain shift according to individual anatomical variation are essential. Postoperative MRI examination is important in the verification of the exact location of the electrode.
Figure 1Misplacement of the right electrode targeted to the anterior nucleus of the thalamus (ANT), within the third ventricle (patient #1). (A) An axial computed tomographic (CT) image showing the location of bilateral electrodes (arrows) targeted to the ANT. It is difficult to confirm the exact location of two, closely situated, metallic artifacts. Arrowheads indicate metallic artifacts from bilateral hippocampal depth electrodes simultaneously implanted during ANT deep brain stimulation. (B) A three-dimensional-reconstructed, coronal CT image showing bilateral electrodes for ANT (arrows). It is difficult to verify the location of electrodes. Arrowheads indicate bilateral hippocampal depth electrodes. (C) A T2-weighted, axial magnetic resonance image (MRI) clearly showing misplacement of the right electrode (arrow) within the ventricle. A small, round, low signal intensity indicates the electrode (arrow). An arrowhead indicates the left electrode within the ANT. (D) A T2-weighted, coronal MRI showing misplacement of the right electrode (arrow) within the ventricle (arrowhead). 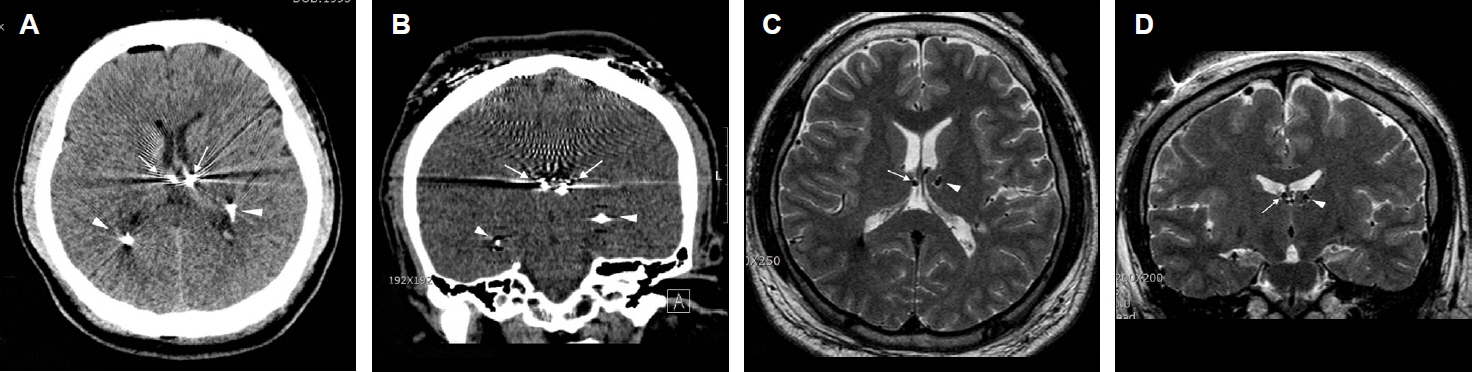
Figure 2Misplacement of bilateral electrodes targeted for the centromedian nucleus (CM) of the thalamus (patient #2). (A) An axial computed tomographic (CT) image showing bilaterally misplaced electrodes aimed for the CM. Note the significant pneumocephalus (arrow) following electrode implantation, indicating an intraoperative brain shift. (B) A coronal magnetic resonance image (MRI) image showing significant asymmetry of the thalamus. The right thalamus is significantly smaller than the left. Arrows indicate the misplaced electrodes. Individual anatomical variation from congenital anomaly and intraoperative brain shift were thought to be the cause of misplacement. 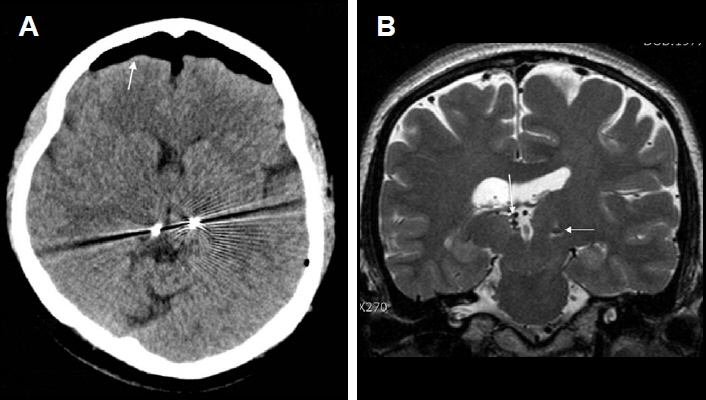
Figure 3Misplacement of left electrode, targeted to the anterior nucleus of the thalamus (ANT), within the third ventricle (patient #6). (A) An axial CT images showing bilateral electrodes for the ANT. It may appear that the electrodes are well placed within the bilateral ANT (left). Arrows indicate transventricular trajectory of electrode implantation (right). (B) Axial (left) and coronal (right) magnetic resonance image (MRI) images showing the location of the bilateral electrodes. The left electrode (arrows) is placed within the lateral and third ventricles. Only the distal two contacts of the right electrode (arrowheads) are placed within the ANT. 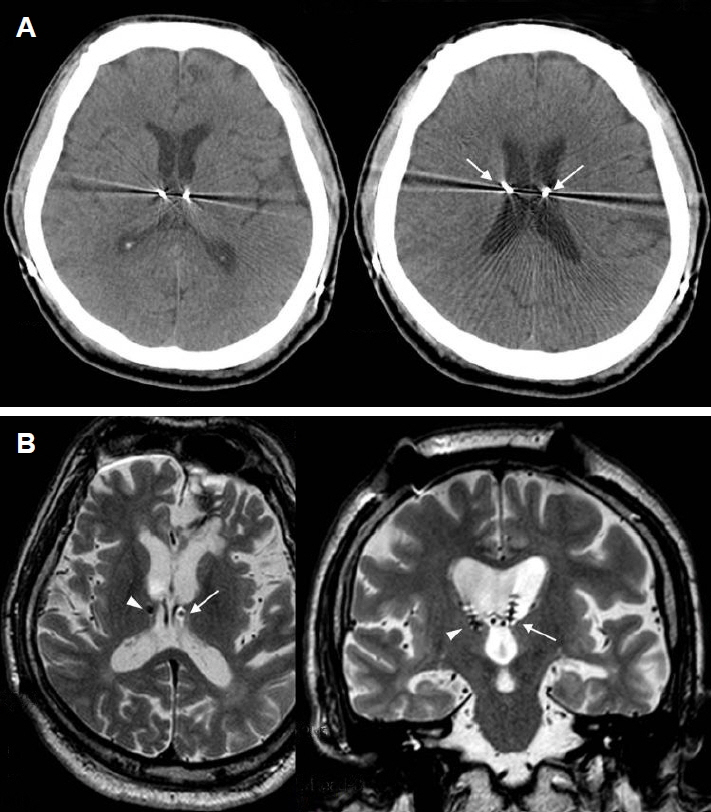
Table 1Demographics and causes of mistargeting in the deep brain stimulation (DBS) of the thalamus EEG, electroencephalography; CT, computed tomography; G/A, general anesthesia; MRI, magnetic resonance imaging; ANT, anterior nucleus of the thalamus; CM, centromedian nucleus; M, male; bil., bilateral; hippo, hippocampus; iCT, intraoperative computed tomography; TLE, temporal lobe epilepsy; DE, depth electrode; Rt., right; DR, driving response; F, female; Lt., left; CPS, complex partial seizure; FLE, frontal lobe epilepsy; yrs, years. Table 2Reported strategies to improve image-guided targeting of the ANT with improved delineation on the MRI
AM, anteromedial subnucleus of ANT; ANT, anterior nucleus of the thalamus; Apr, anterior principal subnucleus of ANT; AV, anteroventral subnucleus of ANT; EML, external medullary lamina; MPRAGE, magnetization-prepared rapid acquisition of gradient echo; MER, microelectrode recording; MTT, mammillothalamic tract; STIR, short tau inversion recovery. References2. Valentín A, Navarrete EG, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54:1823–33.
3. Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in the treatment of intractable seizures. Epilepsia. 1992;33:841–51.
4. Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–25.
5. Lehtimäki K, Möttönen T, Järventausta K, et al. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016;9:268–75.
6. Krishna V, King NK, Sammartino F, et al. Anterior nucleus deep brain stimulation for refractory epilepsy: insights into patterns of seizure control and efficacious target. Neurosurgery. 2016;78:802–11.
7. Velasco F, Velasco M, Velasco AL, Jimemez F, Marquez I, Rise M. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia. 1995;36:63–71.
8. Velasco AL, Velasco F, Jimémez F, et al. Neuromodulation of the cen-tromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–12.
9. Son BC, Shon YM, Choi JG, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. 2016;94:187–97.
10. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908.
11. Zrinzo L, van Hulzen AL, Gorgulho AA, et al. Avoiding the ventricle: a simple step to improve accuracy of anatomical targeting during deep brain stimulation. J Neurosurg. 2009;110:1283–90.
12. Son BC, Shon YM, Kim SH, Choi JG, Kim J. Relationship between postoperative EEG driving response and lead location in deep brain stimulation of the anterior nucleus of the thalamus for refractory epilepsy. Stereotact Funct Neurosurg. 2016;94:336–41.
13. Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–300.
14. Hughes EJ, Bond J, Svrckova P, et al. Regional changes in thalamic shape and volume with increasing age. Neuroimage. 2012;63:1134–42.
15. Wu C, D’Haese PF, Pallavaram S, Dawant BM, Konrad P, Sharan AD. Variations in thalamic anatomy affect targeting in deep brain stimulation for epilepsy. Stereotact Funct Neurosurg. 2016;94:387–96.
16. Möttönen T, Katisko J, Haapasalo J, et al. Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: deliniation using 3 T MRI and intraoperative micro-electrode recording. Neuroimage Clin. 2015;7:823–9.
17. Buentjen L, Kopitzki K, Schmitt FC, et al. Direct targeting of the thalamic anteroventral nucleus for deep brain stimulation by T1-weighted magnetic resonance imaging at 3 T. Stereotact Funct Neurosurg. 2014;92:25–30.
18. Oh YS, Kim HJ, Lee KJ, Kim YI, Lim SC, Shon YM. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. 2012;21:183–7.
19. Velasco F, Velasco M, Jiménez F, et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47:295–304. discussion 304–5.
20. Son BC, Han SH, Choi YS, et al. Transaxillary subpectoral implantation of implantable pulse generator for deep brain stimulation. Neuromodulation. 2012;15:260–6.
21. Morel A. Stereotactic atlas of the human thalamus and basal ganglia. New York: Informa Healthcare USA; 2007.
22. Choi JG, Kim SH, Lee SH, Shon YM, Son BC. Variability of the target coordinates in thalamic deep brain stimulation for epilepsy, review. J Kor Soc Ster Func Neurosurg. 2015;11:53–9.
23. Child ND, Benarroch EE. Anterior nucleus of the thalamus: Functional organization and clinical implications. Neurology. 2013;81:1869–76.
24. Velasco M, Velasco F, Velasco AL, et al. Electrocortical and behavioral responses produced by acute electrical stimulation of the human centromedian thalamic nucleus. Electroencephalogr Clin Neurophysiol. 1997;102:461–71.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||