Introduction
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a well-described clinical disorderin children and adults,1 and is one of the well-known contributors to encephalitis in children.2 This autoimmune disease is becoming increasingly recognized in the pediatric population,3 yet the pathophysiology in most cases of anti-NMDAR encephalitis remains unknown. However, a few cases of this disorder have been thought to have a paraneoplastic origin.4,5 Children with anti-NMDAR encephalitis initially present with prodromal symptoms of neuropsychiatric abnormalities in behavior, speech, and mood, which are often accompanied by personality change, memory loss, and seizures. A definitive diagnosis requires the detection of NMDAR antibodies in the cerebrospinal fluid (CSF).6 Early diagnosis and prompt treatment can improve outcomes; however, there is no consensus guideline for the optimal management of patients with this disease, particularly in children.6,7 Here, we report a case of anti-NMDAR encephalitis in a 13-year-old female treated with rituximab. This case report may contribute towards elucidating the benefits of early intervention using rituximab to improve neurological deficits, reduce the amount of residual anti-NMDAR antibodies in the CSF, and achieve baseline recovery in patients with anti-NMDAR encephalitis, who show only a partial response or are refractory to first-line therapies.
Case
A 13-year-old female was referred to Samsung Seoul Hospital with frequent clonic movements of the right arm and leg followed by right-sided weakness, paresthesia, and acute onset of aphasia. One month prior, she had clonic movements of the right arm and leg lasting about 2 minutes without loss of consciousness. The results of electroencephalography (EEG) and brain magnetic resonance imaging (MRI) were unremarkable at initial presentation. She was prescribed levetiracetam for the prevention of seizures.
Two weeks after the initial presentation, the patient experienced right leg weakness along with right hand and right foot paresthesia and was referred to Samsung Seoul Hospital. Upon admission, the patient experienced fever and her temperature had risen to 38°C. She exhibited clonic movements of the right arm, was inarticulate and could neither verbalize nor understand others.
She was born from non-consanguineous parents at the 38th week of gestation by spontaneous vaginal delivery at an appropriate gestational age. There was no history of significant illness, the results of her general physical examination were normal, and there was no evidence of an external injury. She was alert and the results of her cranial nerve examination were also normal. She exhibited decreased right-sided motor power (motor grade IV) and right-sided paresthesia. There were no pathological reflexes.
Initial clinical course
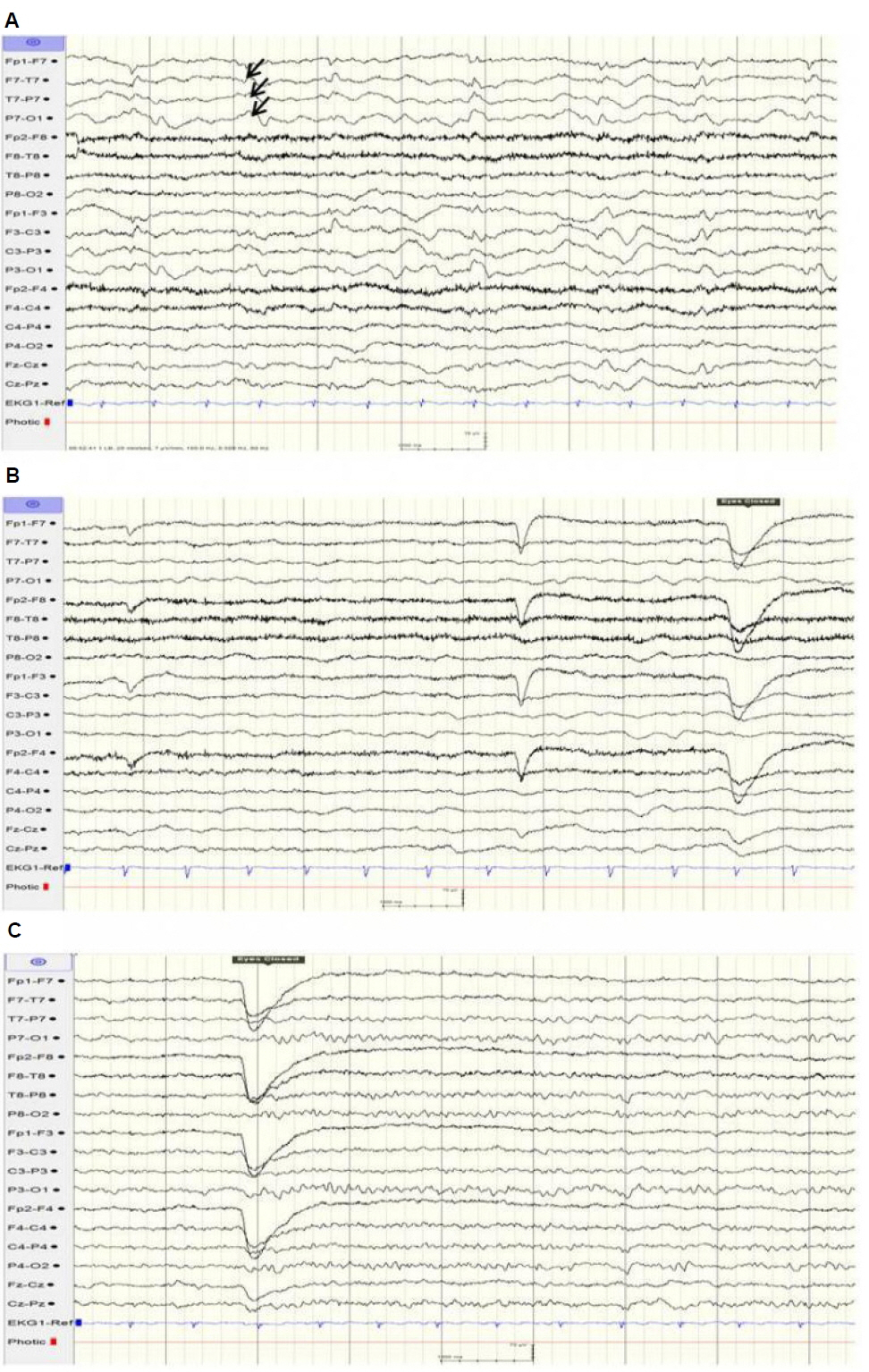
The patient exhibited continuous right-sidedclonic movements at the time of transfer. The clinical impression was an epilepsiapartialis continua because the EEG revealed continuous slow waves in the left hemisphere and frequent spikes or sharp wave discharges from the left temporal areas and identified multiple electroclinical seizures arising from the left temporal area (Fig. 1). The seizures were-controlled with intravenous lorazepam (5 mg). She was admitted to the general ward and treated with antibiotics (cefotaxime, vancomycin, and azithromycin), an antiviral agent (acyclovir), antiepileptic drugs (levetiracetam and oxcarbazepine), and dexamethasone.
Laboratory findings
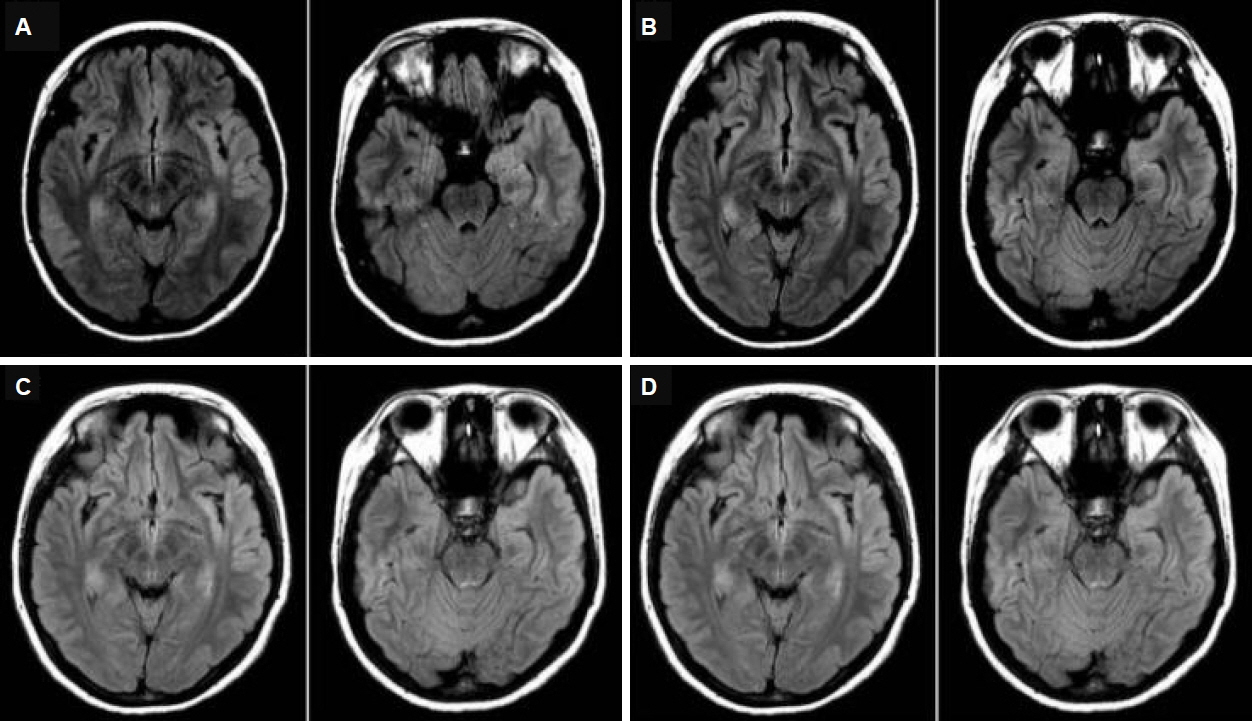
The complete blood count was normal. Antinuclear antibodies, rheumatoid factor, immunoglobulins, complement, thyroid function test, and α-fetoprotein were within normal limits. There was no remarkable elevation of anti-viral antibody titers from serum and CSF samples, including herpes simplex virus, enterovirus, and Japanese B encephalitis virus. The CSF analysis did not indicate pleocytosis or other biochemical abnormalities and was negative for cultures of bacteria and viruses (Table 1). The initial EEG demonstrated electro-clinical seizures arising from the left temporal area. The follow-up EEG revealed intermittent and generalized slow waves, which pertains to diffuse cerebral dysfunction (Fig. 1). T2-weighted brain MRI revealed multifocal high signal intensity lesions and cortical swelling involving bilateral temporal lobes, including the limbic system, which were more promient in the left hemisphere (Fig. 2).
Clinical course during initial admission
The patient was empirically treated for acute bacterial meningitis and acute encephalitis with intravenous vancomycin (60 mg/kg/day divided in 4 doses), cefotaxime (160 mg/kg/day divided in 4 doses), and acyclovir (30 mg/kg/day divided in 3 doses) until the tests for microorganisms yielded results. Additionally, azithromycin (10 mg/kg/day) was administered because mycoplasma pneumoniae infection was suspected, as the mycoplasma pneumoniae antibody titer was 1:640. For the treatment of seizures, diphenylhydantoin (4 mg/kg/day) and levetiracetam (20 mg/kg/day) were administered. From the 2nd hospital day, intravenous immunoglobulin (400 mg/kg/day for 5 days) was introduced considering the differential diagnoses (limbic encephalitis, viral encephalitis, autoimmune encephalitis, and acute disseminated encephalomyelitis) based on brain MRI results. On the 10th hospital day, antibiotic treatments were ceased because the bacterial cultures exhibited no growth of bacterial organisms. Despite the negative polymerase chain reaction results for herpes simplex virus (HSV1 and HSV2), acyclovir was continued for 14 days for the complete treatment of possible herpes virus encephalitis.
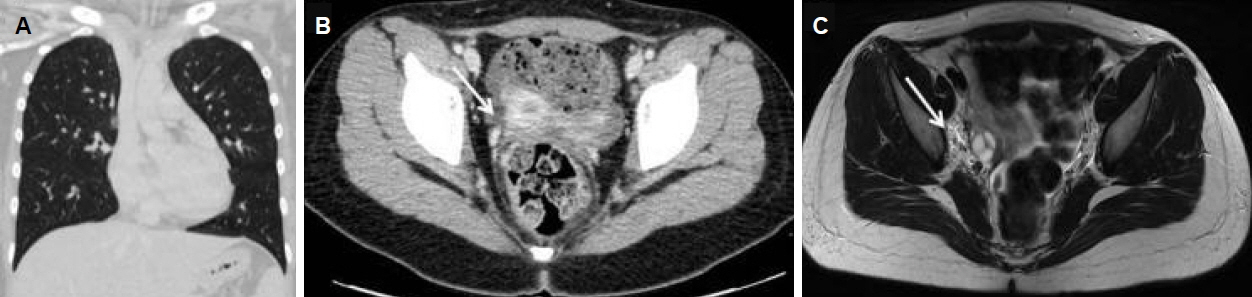
On the 15th hospital day, the CSF tests for autoimmune antibodies revealed the presence of anti-NMDAR antibodies (Table 1). Following this result, methylprednisolone pulse therapy was commenced at 790 mg once daily (15 mg/kg/day) for three consecutive days. To determine the etiology of the anti-NMDAR encephalitis, particularly a possible paraneoplastic origin, chest and abdomen-pelvis computed tomography (CT) scans were performed. On abdomen-pelvis CT, a small, low, attenuated lesion was noted in the right adnexa. For further evaluation, an abdomen-pelvis MRI was performed; the lesion was concluded to be multiple follicles of both ovaries, and there was no demonstrable tumorous lesion (Fig. 3).
Between the 21st and 25th hospital day, there was a significant improvement in the patient’s condition regarding speech, motor power, and paresthesia. The lesions observed on brain MRI decreased in extent, but high signal intensity with cortical swelling involving the temporal lobes and the limbic system, predominantly in the left hemisphere, was still observed on fluid-attenuated inversion recovery (FLAIR). The follow-up EEG on the 21st hospital day revealed continuous slow waves in the left fronto-centro-temporal areas without epileptiform discharges.
The patient was discharged on the 25th hospital day and was prescribed clobazam, valproic acid, diphenylhydantoin, and oxcarbazepine. The patient’s receptivity to communication appeared to be improved, but her speech, particularly word variety, sentence length, and word-finding ability, remained slightly below baseline.
Clinical follow-up after initial admission
By the time of the outpatient follow-up visit, approximately one month after discharge, the patient showed near complete resolution of sensory aphasia, motor weakness, and paresthesia symptoms and a partial resolution of calculation disability and motor aphasia symptoms. However, anti-NMDAR antibodies were still present in the CSF (weakly positive antibody reactivity). Four months after discharge, despite the nearly complete resolution of clinical symptoms, there were no remarkable improvements in brain MRI and EEG results, i.e., no interval change inresidual high signal intensity lesions involving the left temporal lobe, including the limbic system, on brain MRI; and generalized continuous slow waves on the EEG. As a second-line therapy to treat the persistent presence of anti-NMDAR antibodies in the CSF, rituximab (a monoclonal anti-CD20 antibody), 375 mg/m2 per dose weekly was administered four times in total. Eight months after discharge, the patient achieved symptom-free status. The EEG revealed localized intermittent slow waves in the left temporal areas, and the brain MRI indicated decreased extents of high signal intensities in the left temporal lobe (Fig. 2). She was suspected to be at risk of a relapse based on the EEG and the brain MRI results, and a third CSF study was conducted because changes in anti-NMDAR antibody titers in the CSF correlate with clinical relapses.8
At the last visit (24 months after symptom onset and 18 months after rituximab treatment), the patient maintained a seizure-free state and performed well in school, with no demonstrable tumor lesion in the follow-up tumor screening. Therefore, the remaining antiepileptic drug, oxcarbazepine, was discontinued.
Discussion
Anti-NMDAR encephalitis, originally described in 2005 as a para-neoplastic syndrome in a group of women with ovarian teratoma, was linked to the presence of anti-NMDAR antibodies and was officially defined in 2007.5 Approximately 40% of reported patients with anti-NMDAR encephalitis are < 18 years of age.6 A recent study compiling data from more than 577 patients with anti-NMDAR encephalitis found that 37% of them were in the pediatric age group.9 Most children with anti-NMDAR encephalitis present with seizures or movement disorders10 rather than psychiatric symptoms such as anxiety, agitation, paranoia, and visual or auditory hallucinations, which are more predominant in adults with anti-NMDAR encephalitis.5,11
The patient in this study presented with epilepsia partialis continua, which is rarely reported in pediatric anti-NMDAR encephalitis,12,13 along with motor aphasia and unilateral sensory changes. According to a recent study, the first neurological symptoms observed in young children with anti-NMDAR encephalitis included seizure (72%), particularly focal seizure (42%), with a median onset of 15 days before other encephalitis symptoms appeared; other patients mostly exhibited behavioral disorders (26%).14 Thus, in patients presenting a novel onset of altered mental status, seizures, or behavioral changes, anti-NMDAR encephalitis should be systematically considered in the absence of other apparent etiologies.
With early diagnosis and effective treatment, patients with anti-NMDAR encephalitis have a relatively good prognosis and can achieve baseline recovery and complete resolution at follow-up examination.4,15 According to the California Encephalitis Project, the number of young patients with anti-NDMAR encephalitis was greater than that of patients with viral encephalitis.16 Thus, anti-NMDAR encephalitis must be considered and should be part of the differential diagnosis in young patients with limbic encephalitis.
In this case, the EEG revealed regional epileptiform discharges and slow waves, which are frequently described in children with anti- NMDAR encephalitis as well as in adults.17 This patient initially presented unremarkable changes in the brain MRI, but the following MRI (after the presentation of motor aphasia and epilepsiapartialis continua) indicated increased signals on T2-weighted FLAIR MRI sequences involving the temporal lobes, including the limbic system, predominantly in the left hemisphere. The findings of the brain MRI in our patient were consistent with findings of anti-NMDAR encephalitis.12,18 In most cases (89%), brain MRI results are normal or reveal non-specific focal changes at the initial presentation, as in this case.6 Another study reported that most patients with NMDAR encephalitis (66%) had normal brain MRI findings, and the remaining 44% had a wide variation in the distribution and degree of T2-FLAIR hyperintense signal changes throughout the brain.18
The majority of anti-NMDAR encephalitis cases are idiopathic in pathophysiology; however, a significant minority can be attributed to a paraneoplastic origin.19 Regardless of tumor presence, patients with anti-NMDAR encephalitis should be treated with first-line immunotherapy, typically corticosteroids, intravenous immunoglobulin (IVIG), or plasmapheresis. The patients without tumors have an initial response rate of 48%.4 In pediatric patients, first-line immunotherapy is effective in only half of the patients diagnosed with anti-NMDAR encephalitis because pediatric patients are less likely to have causative tumors compared to adults.20 If there is no response to the first-line immunotherapy, rituximab or cyclophosphamide are usually recommended for the treatment of clinical symptoms and prevention of relapse.4,5,10,20,21
In this patient, the anti-NMDAR antibodies in the CSF were present without a causative tumor. After detection of anti-NMDAR antibodies in the CSF, methylprednisolone pulse therapy was added to the initial IVIG treatment considering the incomplete resolution of the clinical symptoms. Although remarkable clinical improvements were observed after high-dose steroid treatment, there were residual anti-NMDAR antibodies in the CSF. After the additional rituximab therapy, the patient no longer reported any symptoms (Fig. 4). Despite a prolonged presence of abnormalities in the brain MRI and EEG, there was no demonstrable tumor lesion in the follow-up tumor screening test. A CSF study was performed because changes in anti-NMDAR antibody titers in the CSF correlate with clinical relapses,8 and anti-NMDAR antibodies were not present in the patient’s CSF.
Among patients with anti-NDMAR encephalitis who do not respond to first-line therapy (i.e., steroids, intravenous immunoglobulin), those receiving second-line immunotherapy (i.e., rituximab) achieve a better outcome than those who do not receive second-line agents.7 Patients receiving second-line immunotherapy also experience fewer relapses than those not receiving such therapy.9 Accumulating data suggest that rituximab is an effective treatment for anti-NMDAR encephalitis in both children and adults.22
Anti-NMDAR-encephalitis is mediated by immunoglobulin G antibodies against the GluN1 subunit of the neuronal NMDA receptor. The inflammatory neuronal dysfunction is thought to be initially reversible but potentially progresses to permanent neuronal destruction if untreated, due to prolonged inflammation and NMDA-mediated glutamate excitotoxicity.18,23 Thus, rituximab may be a favorable treatment option for patients who have residual anti-NMDAR antibodies in the CSF and for those who do not respond to immunosuppressive therapy (i.e., corticosteroids and IVIG).7,24
In summary, this report discusses the case of a 13-year-old female with anti-NMDAR encephalitis, which was idiopathic in etiology. She received first-line (i.e., steroid and intravenous immunoglobulin) and second-line (i.e., rituximab) immunotherapy, and 18 months after initial presentation, normalcerebral function was restored and there were no residual anti-NMDAR antibodies in the CSF. Patients with anti-NMDAR encephalitis typically present with psychiatric symptoms and partial seizures, and it is important that they receive an early diagnosis and prompt treatment for an improved prognosis. The medical history, combined with brain MRI, EEG, and CSF examination, was helpful in the diagnosis of anti-NMDAR encephalitis. Our case suggests that in patients who have residual anti-NMDAR antibodies or in those who do not tolerate first-line immunotherapy, rituximab could be considered as a treatment option. Thus, physicians might consider the early initiation of second-line immunotherapy in certain cases of anti-NMDAR encephalitis.