Introduction
Perampanel is the first selective noncompetitive α-amino-3-hy-droxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist for treating epilepsy.1 Its efficacy and tolerability as an adjuvant treatment in adults with drug-resistant focal seizure were demonstrated in three randomized, double-blind, placebo-controlled studies, and its efficacy as an adjuvant treatment for generalized seizure was also shown in a randomized study.2,3 The usual starting dose of perampanel for focal and generalized seizures in adult patients who are not concomitantly taking an enzyme-inducing antiepileptic drug (AED) is 2 mg/day before sleep. Additionally, it is recommended that the dose be increased by 2 mg/day biweekly to reach the maximum tolerable dose (usually 4–8 mg/day), which provides excellent seizure control. Among the currently available AEDs, perampanel has the longest metabolic half-life, up to 105 hours; thus, a theoretical steady-state can be reached in approximately 2–3 weeks after the drug is introduced or the dose adjusted.4
Perampanel treatment can be associated with various adverse effects. While the occurrence of psychiatric reactions is the most troublesome and often hinders the drug’s widespread use in epilepsy patients, adverse dose-dependent central nervous system (CNS) effects such as dizziness, somnolence, and fatigue are the most frequently observed. Therefore, severe adverse CNS effects may occur in patients with acute perampanel overdose and could be prolonged given the drug’s long half-life. However, there is currently no report on the acute overdose of perampanel in epilepsy patients. We recently treated a patient who took 10 times the usual perampanel maintenance dose in an attempted suicide.
Case
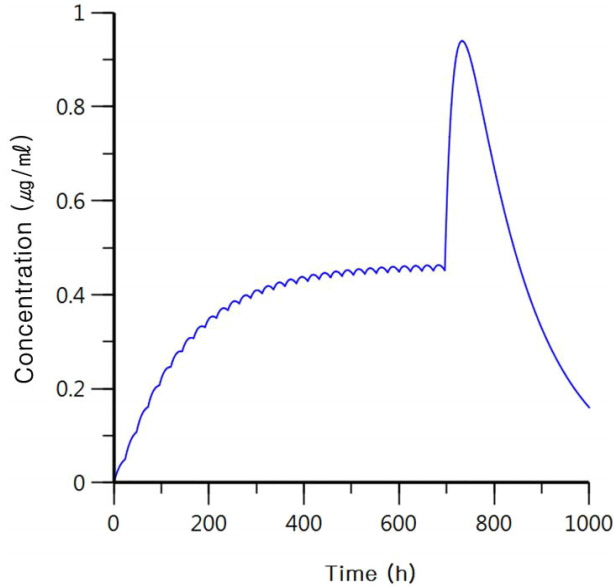
A 39-year-old woman with an unremarkable medical history visited our neurology department complaining of recurrent seizures. Magnetic resonance imaging showed non-enhancing diffuse enlargement of the right amygdala, hippocampus, and parahippocampal gyrus, and electroencephalography showed occasional spikes in corresponding areas. The patient was initially prescribed 1,000 mg of val-proic acid once a day before sleep, but her seizures persisted. Two mg/day of perampanel before sleep was added as adjunctive therapy and up-titrated to 4 mg/day after 2 weeks. Seizures were controlled with the combination therapy of 4 mg/day of perampanel and 1,000 mg/day of valproic acid. After 11 months without seizures, the patient was admitted to the intensive care unit for altered consciousness. Her records documented that she had recently episode had a depression because of job loss and that she had taken 10 times the daily dosage of the prescribed AEDs (40 mg of perampanel and 10,000 mg of valproic acid) in a suicide attempt. On neurological examination, her mental status was stuporous, but her reflexes were normal and there were no focal neurological signs or symptoms. Radiological evaluations, including brain computed tomography and diffusion-weighted magnetic resonance imaging, showed no abnormality, and continuous electroencephalography monitoring showed continuous low-amplitude slow activity without epileptiform discharges. Her blood valproic acid level on hospital day 2 exceeded 150 μg/mL and decreased to 88.39 and 3.44 μg/mL on day 3 and 7, respectively. The patient’s blood ammonia level on hospital day 3 was 125.2 μg/dL and decreased to 53.7 μg/dL, within the reference range. Because it was impossible to measure the blood level of perampanel, we attempted to predict the patient’s recovery time based on the known pharmacokinetics of per-ampanel (clearance, 0.75 L/h; volume of distribution, 100 L; absorption rate constant, 0.005 h−1). We assumed that perampanel was regularly administered at a dosage of 4 mg/day for 28 days to reach the steady-state level and that the 40 mg had been taken at the 696th hour (day 29). We calculated that the blood perampanel level would increase up to twice the usual steady-state level but would decrease to the steady-state level at day 36 (7 days after she took 40 mg of the drug; Fig. 1).
During the patient’s hospital stay, vital signs and other laboratory results remained stable during the first 2 days, and there was no evidence of perampanel-induced systemic adverse effects such as hepatic or renal toxicity, hypotension, or respiratory suppression. However, we noted sudden respiratory difficulty on hospital day 3, and laboratory studies showed blood D-dimer levels exceeding 20 μg/mL and arterial hypoxia. Chest computed tomography also showed the development of a widespread pulmonary embolism. Under treatment with intravenous heparin anticoagulation and supportive mechanical ventilation, the patient began to regain consciousness on hospital day 6, returning to her baseline mental status on day 8. Due to the possible adverse psychiatric effect of per-ampanel, which may have been associated with the suicide attempt, we recommended changing to another AED during the patient’s hospital stay. However, the patient denied any causal relationship between the drug and the attempt and insisted that perampanel was effective for seizure control. At the 4-month follow-up at the out-patient clinic, she had remained seizure free with 4 mg/day of per-ampanel and 100 mg/day of valproic acid.
Discussion
We report here a patient who had a severe overdose of per-ampanel, 10 times the usual maintenance dose. Considering that the usual maintenance dose is 4–8 mg/day and the upper limit of the recommended daily dose is 12 mg/day,4 our patient had been administered a relatively low dose (4 mg/day), and the dose she accidentally took (40 mg/day) was only 3.3 times higher than the upper limit of the recommended daily dose. However, it took approximately 1 week for her to recover consciousness, and this prolonged adverse CNS effect was likely associated with perampanel’s long half-life. In previous reports of patients who had intoxication with other AEDs, systemic toxicity, including hyponatremia from oxcarbazepine, and cardiac arrhythmia from phenytoin, was occasionally observed, but patients recovered 2 or 3 days after admission.5,6 Our report shows that acute perampanel overdose may not produce serious adverse systemic effects, such as cardiac toxicity, respiratory depression, or other metabolic derangements, but that adverse CNS effects can be prolonged and that the recovery of consciousness may take longer in patients with high intoxication doses of the drug.
Psychiatric reactions are well-noted and troublesome adverse events of perampanel, and patients with perampanel treatment should be monitored for the occurrence of psychiatric reactions. Suicidal ideation and behavior have been reported in patients treated with AEDs on several occasions. A specific role of perampanel treatment in neuropsychiatric adverse effects is also suggested by observational studies. In a study of 47 individuals treated with a median perampanel dose of 8 mg/day (range, 2–12 mg/day), behavioral alterations were the most frequent reason for discontinuation of treatment. In this cohort, three patients were reported to behave aggressively or experience suicidality.7
One remarkable finding was that our patient had pulmonary embolism during admission that necessitated anticoagulation therapy and mechanical ventilation. This was unexpected because AMPA receptors play an important role in modulating platelet activation and thrombosis, and AMPA receptor antagonists have been suggested for treating or preventing stroke, myocardial infarction, and other thrombotic diseases.8–11 The prolonged immobilization during hospital stay have been the main cause of pulmonary embolism in our patient, but it can also be speculated that the high blood level of perampanel during the acute overdose blocked the usual platelet activation; this might have induced a hypocoagulable state, and withdrawing the AMPA antagonist might have induced a transient hypercoagulable state that produced the embolism as a result of immobilization. In summary, these findings show that perampanel overdose can produce prolonged stupor due to its long half-life, and that caution should be exercised regarding the transient hypercoagulable state that could be associated with perampanel’s effect on AMPA receptors.