AbstractThe resective epilepsy surgery can be the effective procedure to get seizure-free outcome in these drug resistant epilepsy (DRE) patients. Class I evidence firmly establishes the superiority of epilepsy surgery over medical treatments in both seizure control and quality of life for DRE patients. For the effective identification of optimal surgical candidates, it's essential to understand the prognostic factors of epilepsy surgery based on the surgical methods employed. Established positive prognostic indicators for temporal resection include the presence of hippocampal sclerosis on magnetic resonance imaging (MRI), focal lesions on MRI, unilateral temporal spikes, concordant ictal electroencephalography (EEG), and a history of prolonged febrile convulsion. Potential negative predictors encompass preoperative secondary generalized tonic-clonic seizures, a normal MRI, postoperative EEG spikes, and age at the time of surgery. For neocortical epilepsy, the prognostic factors identified through multivariate analysis were the presence of a discrete lesion, localized hypometabolism on Fluorodeoxyglucose positron emission tomography (FDG-PET), and localized ictal EEG. A significant correlation was found between achieving a seizure-free outcome in no visible lesion on MRI (MR-negative) epilepsy patients and having concordance in two or more presurgical evaluations, specifically in interictal EEG, ictal EEG, FDG-PET, and ictal single-photon emission computed tomography. There was a marked improvement in the seizure-free outcome in MR-negative temporal lobe epilepsy (TLE) by the application of this strategy. The better surgical candidates for epilepsy surgery are the followings: patients displaying a discrete lesion on MRI with concordant video-EEG monitoring (VEM) results, patients diagnosed with unilateral hippocampal sclerosis who have concordant VEM results, patients with unilateral hippocampal sclerosis but discordant VEM results, patients with focal cortical dysplasia and concordant VEM results, and patients diagnosed with MR-negative TLE who exhibit two or more consistent results from presurgical evaluations.
IntroductionMany patients with epilepsy respond favorably to antiseizure medications (ASMs).1 Over 50.0% of those with focal epilepsy achieve seizure freedom with a single ASM. However, there are individuals who remain resistant to seizures even when prescribed multiple ASMs in combination. The International League Against Epilepsy defines "seizure freedom" as the absence of seizures for at least three times the longest pre-intervention inter-seizure interval or for 12 months, whichever is longer.2 Drug-resistant epilepsy (DRE) is characterized by the failure of two appropriately selected and tolerated ASMs to maintain seizure freedom. A recent systematic review and meta-analysis determined that the prevalence of DRE is 13.7% in population or community-based studies, and 36.3% in clinic-based cohorts. A staggering 75.0% of all epilepsy care costs are allocated to the treatment and care of DRE patients.
DRE is typified by persistent seizures, an excessive load of ASMs, cognitive decline due to neurobiochemical changes, associations with psychosocial dysfunction, dependent behaviors, a limited life-style, diminished quality of life (QOL), and an elevated risk of mortality from accidents or sudden unexpected death in epilepsy (SUDEP).3 Resective epilepsy surgery has emerged as an effective treatment for achieving seizure freedom in patients with DRE. To optimize the benefits of epilepsy surgery, it is essential to confirm the intractability of a patient's condition and demonstrate that surgery is superior to medical treatment in terms of achieving both seizure freedom and enhanced QOL. The choice of surgical procedure should be informed by a thorough presurgical evaluation. It is crucial that suitable candidates receive epilepsy surgery without unnecessary delay and that the surgery is not underutilized. For optimal surgical outcomes, guidelines for identifying the most promising surgical candidates are indispensable.
Superiority of Surgery over Medical Treatment for DRE PatientsFor the treatment of DRE patients, it's essential to have robust clinical evidence supporting the advantages of epilepsy surgery over medical interventions. Recent randomized controlled trials focused on DRE have underscored the superiority of surgical approaches compared to ASMs.
In a notable study,4 80 patients with temporal lobe epilepsy (TLE) were divided equally between surgical intervention and medical treatment for a duration of 1 year. At the 1-year mark, 58.0% of the surgical cohort achieved seizure freedom, in contrast to only 8.0% in the medical group (p<0.001). Notably, patients who underwent surgery reported an improved QOL.
Another study-this one being a multicenter, controlled, parallel-group trial-sought to validate the efficacy of early epilepsy surgery for drug-resistant TLE patients.5 The study comprised 39 patients with mesial TLE (mTLE) who had been experiencing disabling seizures for a maximum of two consecutive years despite two adequate ASM trials. During the second year of follow-up, none of the 23 participants in the medical group were seizure-free, while 11 out of the 15 in the surgical group achieved this outcome. Furthermore, the surgical cohort showcased a more significant average improvement in QOL.
An additional study assessed epilepsy surgery's efficacy for children and adolescents diagnosed with DRE, compared to medical treatment (n=116).6 Surgical interventions encompassed temporal resection, extratemporal resection, hemispherectomy, corpus callosotomy, and resection of hypothalamic hamartoma. A year post-surgery, 77.0% of the surgical group were free from seizures, as opposed to a mere 7.0% in the medical group. Moreover, the surgical group demonstrated enhanced behavioral scores and QOL relative to their medical counterparts.
In conclusion, class I evidence firmly establishes the superiority of epilepsy surgery over medical treatments in both seizure control and QOL for DRE patients. Additionally, a noteworthy decrease in healthcare costs was observed following successful epilepsy surgery.
Recognition of Surgically Remediable Epilepsy SyndromesIt's crucial to identify surgical candidates at an early stage to prevent lifelong dependence on family and society. Delayed recognition can result in developmental setbacks and behavioral disturbances. Common surgically remediable epilepsy syndromes include mTLE with hippocampal sclerosis, DRE with a discrete focal lesion visible on magnetic resonance imaging (MRI), and diffuse hemispheric pathology.7,8 However, many patients might not fit these specific criteria but are still viable candidates for surgery.
The objectives of epilepsy surgery encompass achieving a lifelong seizure-free status, avoiding surgical complications, and enhancing the QOL. The criteria for considering epilepsy surgery have evolved.9,10 In the past, the experience of four or more complex partial seizures (focal seizures with impaired consciousness) was a guideline for considering surgical intervention. Today, individuals who experience one or even fewer seizures that disrupt everyday life monthly might be evaluated for epilepsy surgery. The extent to which a seizure impedes daily activities has become a pivotal factor in deciding on surgical intervention. Cost-effectiveness, reduced morbidity from surgery, and increased mortality risks from accidents and SUDEP all advocate for a broader application of epilepsy surgery.
For the effective identification of optimal surgical candidates, it's essential to understand the prognostic factors of epilepsy surgery based on the surgical methods employed. Established positive prognostic indicators for temporal resection include the presence of hippocampal sclerosis on MRI, focal lesions on MRI, unilateral temporal spikes, concordant ictal electroencephalography (EEG), and a history of prolonged febrile convulsion.7,8,11–15 Potential negative predictors encompass preoperative secondary generalized tonic-clonic seizures (2GTCS), a normal MRI, postoperative EEG spikes, and age at the time of surgery. Earlier intervention typically forecasts a more favorable outcome, which could be attributed to the progressive nature of mTLE.
A systematic review and meta-analysis examining long-term seizure outcomes after epilepsy surgery determined that the median seizure-free rate was 66.0% for temporal resections, 36.0% for occipital and parietal resections, and 27.0% for frontal resections.14 Another meta-analysis, which reviewed 47 articles to identify predictors of epilepsy surgery outcomes, found positive predictors to include a history of febrile convulsions, the presence of a tumor, abnormal MRI findings, the presence of hippocampal sclerosis, concordance between MRI and EEG, and extensive surgical resection.16 Negative predictors were the presence of postoperative abnormal EEG discharges and the need for intracranial monitoring. For neocortical epilepsy, the prognostic factors identified through multivariate analysis were the presence of a discrete lesion, localized hypometabolism on Fluorodeoxyglucose positron emission tomography (FDG-PET), and localized ictal EEG.11 A multicenter, prospective observational study found that 223 out of 339 patients (66.0%) achieved a 2-year seizure remission, with no significant difference observed between medial temporal and neocortical resections.17 The only positive predictors were the absence of 2GTCS and the presence of hippocampal sclerosis on MRI. Most patients underwent medial temporal resection (297 patients). In cases with tumors, epileptogenic lesions are more readily detected and typically present a more circumscribed pathology. In contrast, focal cortical dysplasia (FCD) tends to be more extensive than what's revealed by MRI.18,19 Mild pathological characteristics and incomplete resections are considered poor prognostic factors for FCD.20
MR-Negative (No Visible Lesion on MRI) EpilepsyThe prognosis for epilepsy surgery in patients with MR-negative epilepsy has generally been less than ideal. Seizure-free outcomes vary between 31.0% and 70.0% for MR-negative TLE, and between 17.0% and 57.0% for extratemporal MR-negative epilepsy (Table 1).19–26 Two extensive studies indicated that seizure-free outcomes stand at 47.0% and 55.0%, respectively, for MR-negative TLE, and 41.0% and 43.0% for patients with MR-negative extratemporal lobe epilepsy.25,26
A significant correlation was found between achieving a seizure-free outcome and having concordance in two or more presurgical evaluations, specifically in interictal EEG, ictal EEG, FDG-PET, and ictal single photon emission tomography (SPECT) (Table 2).24 After implementing a new strategy, which considered patients with two or more consistent results across evaluations, there was a marked improvement in the seizure-free outcome post-surgery, especially in MR-negative TLE, with rates rising from 58.1% to 80.6% (Table 3).27
Special Situations in TLEFor patients presumed to have TLE based on presurgical evaluations yet displaying a normal MRI, three different scenarios may arise: mTLE with a normal MRI, neocortical TLE, or extratemporal lobe epilepsy that mimics TLE. Distinguishing mTLE from neocortical TLE with certainty is challenging.28,29 It's widely recognized that patients with medial TLE have heightened epileptogenicity in the hippocampus, and the epileptogenic network of medial TLE has been extensively studied. On the other hand, identifying the epileptogenic zone in neocortical epilepsy is intricate due to the heterogeneity of the affected area and the inherent pathology of neocortical epilepsy. Neocortical epilepsy appears to possess a notably varied epileptogenic network and propagation pathways, even when the epileptogenic zones are similarly located. A deeper comprehension of the epileptogenic network of neocortical epilepsy could lead to improved surgical outcomes.
Currently, pinpointing the epileptogenic zone in presumed TLE without MRI-detected lesions largely depends on intracranial EEG findings. Proper placement of intracranial electrodes on neighboring structures is essential. In such cases, surgical intervention typically involves a tailored resection, which might encompass the temporal pole and mesial temporal structures. The posterior boundaries of the temporal lobe can differ, so surgical decisions should be directed by insights from intracranial electrodes. For some individuals, surgical excision has expanded beyond the temporal lobe to involve areas like the orbitofrontal cortex, frontal pole, prefrontal cortex, parietal operculum, inferior parietal cortex, or the temporo-occipital junction.30–32
Temporal plus epilepsy (TPE) is a term recently coined to describe an epilepsy syndrome where the patient experiences specific seizure types originating from multiple lobes.31 These seizures form a complex epileptogenic network that includes the temporal lobe and adjacent structures, such as the orbitofrontal area, insula, frontal and parietal operculum, and the temporo-parieto-occipital junction.32,33 The existence of extratemporal lobe epilepsy that mimics TLE and TPE may partly account for the comparatively lower success rates of surgical outcomes in MR-negative TLE.
The term "dual pathology" in TLE denotes a situation where a TLE patient exhibits two or more distinct pathological abnormalities.34,35 These multiple factors or abnormalities in the brain play a role in initiating or sustaining epileptic seizures. Dual pathology is a crucial factor to consider during the evaluation and treatment of TLE because it can impact the success and potential risks of resective epilepsy surgery. Prior to surgery, it's essential to address certain questions: are both pathologies contributing to the onset of epilepsy, or is one predominately responsible? Can both be addressed surgically, or is one more suitable for surgical intervention than the other?
Algorithm of Epilepsy SurgeryWhen patients are diagnosed with DRE, it's necessary to contemplate epilepsy surgery (Fig. 1). If a patient displays unilateral hippocampal sclerosis and video-EEG monitoring (VEM) results that align, early surgery is advisable given the natural progression and chronic nature of the condition. For those with bilateral hippocampal sclerosis or inconclusive VEM results alongside unilateral hippocampal sclerosis, stereo-EEG might be required before proceeding with surgery.
In cases where there's a focal lesion evident on MRI that matches VEM results, a straightforward lesionectomy might suffice to manage DRE. However, the areas surrounding arteriovenous malformations or cavernous angiomas tend to be highly epileptogenic due to the deposit of hemosiderin.36 As such, both lesionectomy and marginectomy are necessary. For patients with FCD, there are instances where the periphery of the FCD isn't visible on MRI, and seizures can emanate from this hidden area. Under such circumstances, intracranial EEG, which includes stereo EEG, can be vital to enhancing the surgical outcome.
If a patient has a normal MRI but displays two or more consistent results from FDG-PET, ictal SPECT, interictal EEG, and ictal EEG-indicating MR-negative TLE-surgery is often advised due to its promising outcomes. In these scenarios, intracranial EEG with stereo EEG, covering both the temporal lobe and surrounding areas, becomes imperative.37,38
Conclusion: Better Candidates for Epilepsy SurgeryThe following individuals are deemed most suitable for epilepsy surgery. 1) Patients displaying a discrete lesion on MRI with concordant VEM results. 2) Patients diagnosed with unilateral hippocampal sclerosis who have concordant VEM results. 3) Patients with unilateral hippocampal sclerosis but discordant VEM results (intracranial EEG is necessary). 4) Patients with focal cortical dysplasia and concordant VEM results (intracranial EEG might be required). And 5) patients diagnosed with MR-negative temporal lobe epilepsy who exhibit two or more consistent results from presurgical evaluations (intracranial EEG is essential).
Figure 1Algorithm of eilepsy surgery. TLE, temporal lobe epilepsy; ASM, aniseizure medication; VNS, vagus nerve stimulation; DBS, deep brain stimulation. 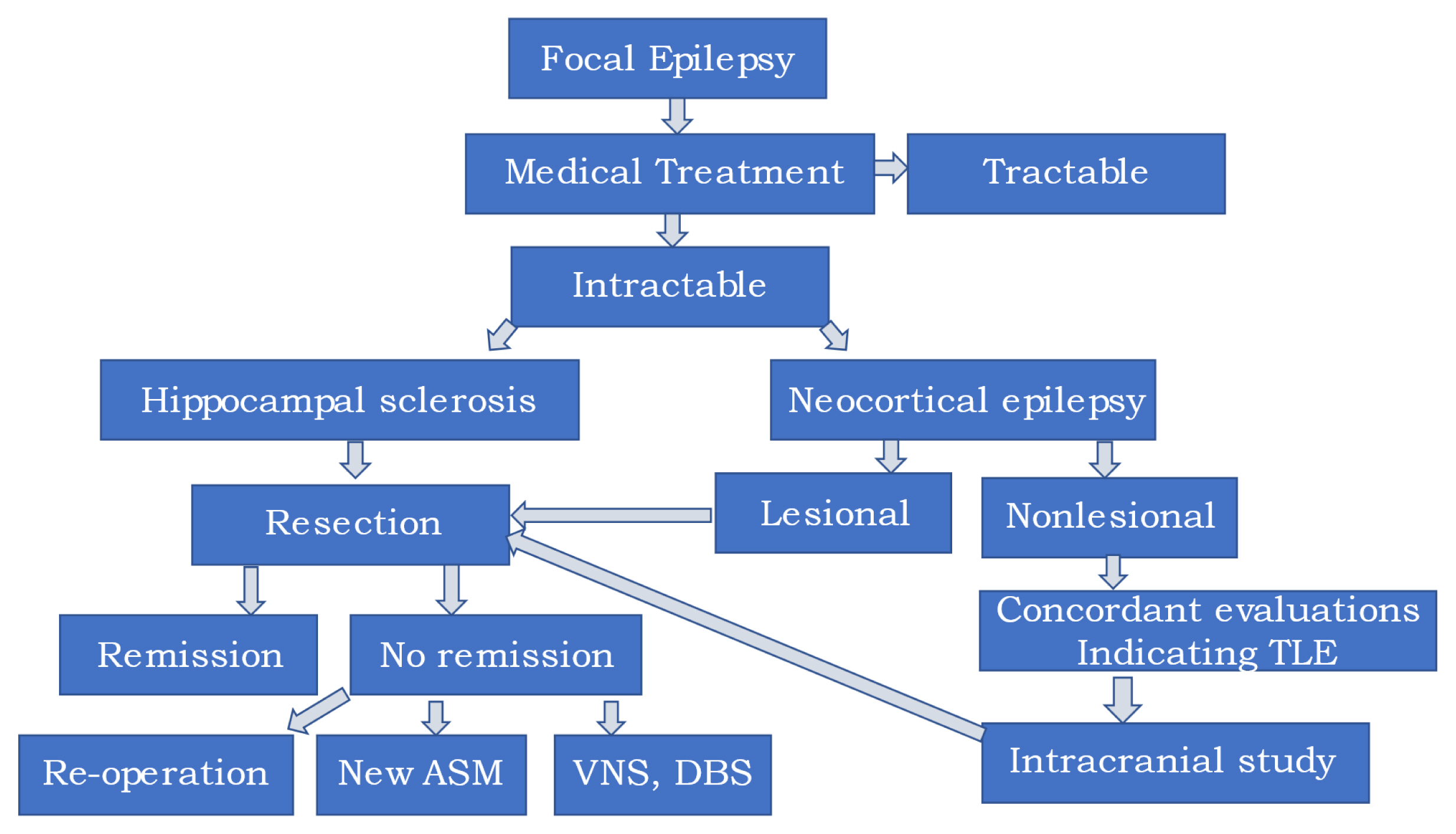
Table 1Surgical outcomes of MR-negative epilepsy according to the various studies
Table 2Concordance rate of presurgical evaluations (FDG-PET, ictal SPECT, interictal EEG, ictal EEG) and surgical outcome of MR-negative neocortical epilepsy
Table 3Seizure-free rate among patients with two or more concordant results. In the new group, the strategy of recruiting DRE patients with two or more concordant results from presurgical evaluations has been implemented
References1. Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54.
2. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51:1069–77.
3. Yoo JY, Panov F. Identification and treatment of drug-resistant epilepsy. Continuum (Minneap Minn). 2019;25:362–80.
4. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8.
5. Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–30.
6. Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377:1639–47.
8. West S, Nolan SJ, Newton R. Surgery for epilepsy: a systematic review of current evidence. Epileptic Disord. 2016;18:113–21.
9. Kwon CS, Neal J, Telléz-Zenteno J, et al. Resective focal epilepsy surgery - has selection of candidates changed? A systematic review. Epilepsy Res. 2016;122:37–43.
10. Culler GW, Jobst BC. Surgical treatments for epilepsy. Continuum (Minneap Minn ). 2022;28:536–58.
11. Yun CH, Lee SK, Lee SY, Kim KK, Jeong SW, Chung CK. Prognostic factors in neocortical epilepsy surgery: multivariate analysis. Epilepsia. 2006;47:574–9.
12. Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia. 2005;46:1273–9.
13. Delev D, Oehl B, Steinhoff BJ, et al. Surgical treatment of extratemporal epilepsy: results and prognostic factors. Neurosurgery. 2019;84:242–52.
14. Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–98.
15. Baciu M, O'Sullivan L, Torlay L, Banjac S. New insights for predicting surgery outcome in patients with temporal lobe epilepsy. A systematic review. Rev Neurol (Paris). 2023;179:607–29.
16. Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87.
17. Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65:912–8.
18. Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y. Focal neuronal migration disorders and intractable partial epilepsy: results of surgical treatment. Ann Neurol. 1991;30:750–7.
19. Blumcke I, Russo GL, Najm I, Palmini A. Pathology-based approach to epilepsy surgery. Acta Neuropathol. 2014;128:1–3.
20. Kim DW, Lee SK, Chu K, et al. Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology. 2009;72:211–6.
21. Siegel AM, Jobst BC, Thadani VM, et al. Medically intractable, localization-related epilepsy with normal MRI: presurgical evaluation and surgical outcome in 43 patients. Epilepsia. 2001;42:883–8.
22. Blume WT, Ganapathy GR, Munoz D, Lee DH. Indices of resective surgery effectiveness for intractable nonlesional focal epilepsy. Epilepsia. 2004;45:46–53.
23. Chapman K, Wyllie E, Najm I, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710–3.
24. Alarcón G, Valentín A, Watt C, et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry. 2006;77:474–80.
25. Jayakar P, Dunoyer C, Dean P, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49:758–64.
26. Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32.
27. Moon HJ, Kim DW, Chung CK, et al. Change of patient selection strategy and improved surgical outcome in MRI-negative neocortical epilepsy. J Epilepsy Res. 2016;31:66–74.
28. O'Brien TJ, Kilpatrick C, Murrie V, Vogrin S, Morris K, Cook MJ. Temporal lobe epilepsy caused by mesial temporal sclerosis and temporal neocortical lesions. A clinical and electroencephalographic study of 46 pathologically proven cases. Brain. 1996;119:2133–41.
29. Gil-Nagel A, Risinger MW. Ictal semiology in hippocampal versus extra-hippocampal temporal lobe epilepsy. Brain. 1997;120:183–92.
30. Widdess-Walsh P, Jeha L, Nair D, Kotagal P, Bingaman W, Najm I. Subdural electrode analysis in focal cortical dysplasia: predictors of surgical outcome. Neurology. 2007;69:660–7.
31. Spencer SS, Lamoureux D. Invasive electroencephalography evaluation for epilepsy surgery. Shorvon S, Dreifuss F, Fish D, Thomas D, editors. The treatment of epilepsy. 1st ed. Oxford: Blackwell Science; 1996. p. 562–88.
32. Barba C, Barbati G, Minotti L, Hoffmann D, Kahane P. Ictal clinical and scalp-EEG findings differentiating temporal lobe epilepsies from temporal 'plus' epilepsies. Brain. 2007;130:1957–67.
33. Ryvlin P, Kahane P. The hidden causes of surgery-resistant temporal lobe epilepsy: extratemporal or temporal plus? Curr Opin Neurol. 2005;18:125–7.
34. Salanova V, Markand O, Worth R. Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand. 2004;109:126–31.
35. Ho SS, Kuzniecky RI, Gilliam F, Faught E, Morawetz R. Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology. 1998;50:748–54.
36. Wang X, Tao Z, You C, Li Q, Liu Y. Extended resection of hemosiderin fringe is better for seizure outcome: a study in patients with cavernous malformation associated with refractory epilepsy. Neurol India. 2013;61:288–92.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||