Alternate Seizure Spread with Agenesis of the Corpus Callosum
Article information
Abstract
Agenesis of the corpus callosum is a brain malformation that can occur in isolation or in conjunction with other congenital or developmental defects. The clinical sequelae of this condition include epilepsy, cognitive deficits, developmental delay, and various neurological and psychiatric disorders. Here we present the case of a patient with congenital complete agenesis of the corpus callosum and medically refractory epilepsy who underwent stereoelectroencephalography. This identified a left frontal ictal focus and revealed that contralateral spread occurred though the anterior commissure, a rare and interesting occurrence. Left frontal resection resulted in significant improvement. This demonstrates the role of the anterior commissure in ictal spread and the potential for novel methods of seizure spread in patients with temporal lobe epilepsy that must be considered in a surgical approach.
Introduction
Agenesis of the corpus callosum (ACC) is a malformation in which there is partial or complete absence of the corpus callosum. This disorder is the most common cerebral malformation, and the incidence of ACC agenesis has been reported to be between 0.05 and 0.7%.1 Genetic studies have revealed various chromosomal abnormalities and mutations that may play a role in ACC.2 The heterogeneity of presentation as well as many common co-morbities seen in ACC patients make it difficult to identify a specific genetic cause.3 In addition, environmental factors such as alcohol consumption and recreational drug use during pregnancy may play a factor in the development of this condition.4,5
ACC can be an isolated finding or appear in conjunction with other developmental or congenital disorders. One study by Romaniello et al.,6 identified schizencephaly and polymicrogyria as the most common associated cerebral malformations. Brainstem involvement and urological malformations are also seen but are less common. Isolated ACC has been shown to be associated with better clinical outcomes, with complete agenesis rather than partial, showing the best profile.6 ACC can be asymptomatic at one extreme, with individuals displaying essentially normal cognitive and social skills, and at the other extreme is associated with severe cognitive deficits, impairments in gross and fine motor control, and speech, and epilepsy.7,8 Cognitive deficits have been reported to encompass broad defects in complex intellectual functions, social function, and emotional processing.8,9
The corpus callosum is a common pathway through which seizures spread from one hemisphere to another. As such, corpus callosotomy is an available treatment for intractable, multifocal, or diffuse epilepsies.10 Studies have shown the effectiveness of corpus callosotomy in treating tonic and atonic seizures as well as secondarily generalized epilepsies, Lennox-Gastaut syndrome, and West syndrome, where the procedure resulted in the majority of patients experiencing disruption of generalized discharges.10 Secondarily generalized seizures can also be seen in patients with partial or complete ACC, indicating that alternative pathways exist for seizure spread.11
Additional pathways such as the hippocampal commissure and anterior commissure have been posited as methods for seizure spread.12,13 We present here a patient with drug-resistant epilepsy who underwent invasive monitoring with stereoelectroencephalography (SEEG) which revealed focal left frontal onset with rapid spread to the right hemisphere through the anterior commissure, showing a novel route of seizure spread that is rarely reported.
Case Report
This patient was first seen at our institution at the age of 18 years. She had been previously diagnosed with ACC and had severe developmental delay, with an IQ of 44. She had been experiencing refractory seizures since the age of 6 years despite treatment with multiple anti-epileptic drugs and had undergone vagus nerve stimulator (VNS) implantation 3 years before. Due to lack of improvement, the VNS had been turned off 1 year after implantation. Her most common seizure type was a focal aware seizure in which she would grasp her left hip with her left hand while elevating her right hand. These lasted 15 seconds and were triggered by startle, occurred 1–3 times/day, and would cause the patient to fall suddenly if standing, leading to injuries. She had had simple partial status epilepticus twice, both following a prolonged seizure-free period, beginning in a similar fashion as her typical seizures. She also had focal to bilateral tonic-clonic seizures, lasting 1 to 2 minutes, with postictal left facial droop, confusion, and fatigue lasting 30 minutes, although these only occurred three times in her life and were associated with preceding illness or vomiting. Lastly, her family reported tremulous shaking episodes from age 3 to 6, which were attributed to separation anxiety and had not recurred. The patient had severe cognitive impairment with inability to perform simple calculations and could only follow simple one-step commands that did not cross the midline. The rest of the exam was normal. She was taking carbamazepine, lacosamide, topiramate, and fluoxetine.
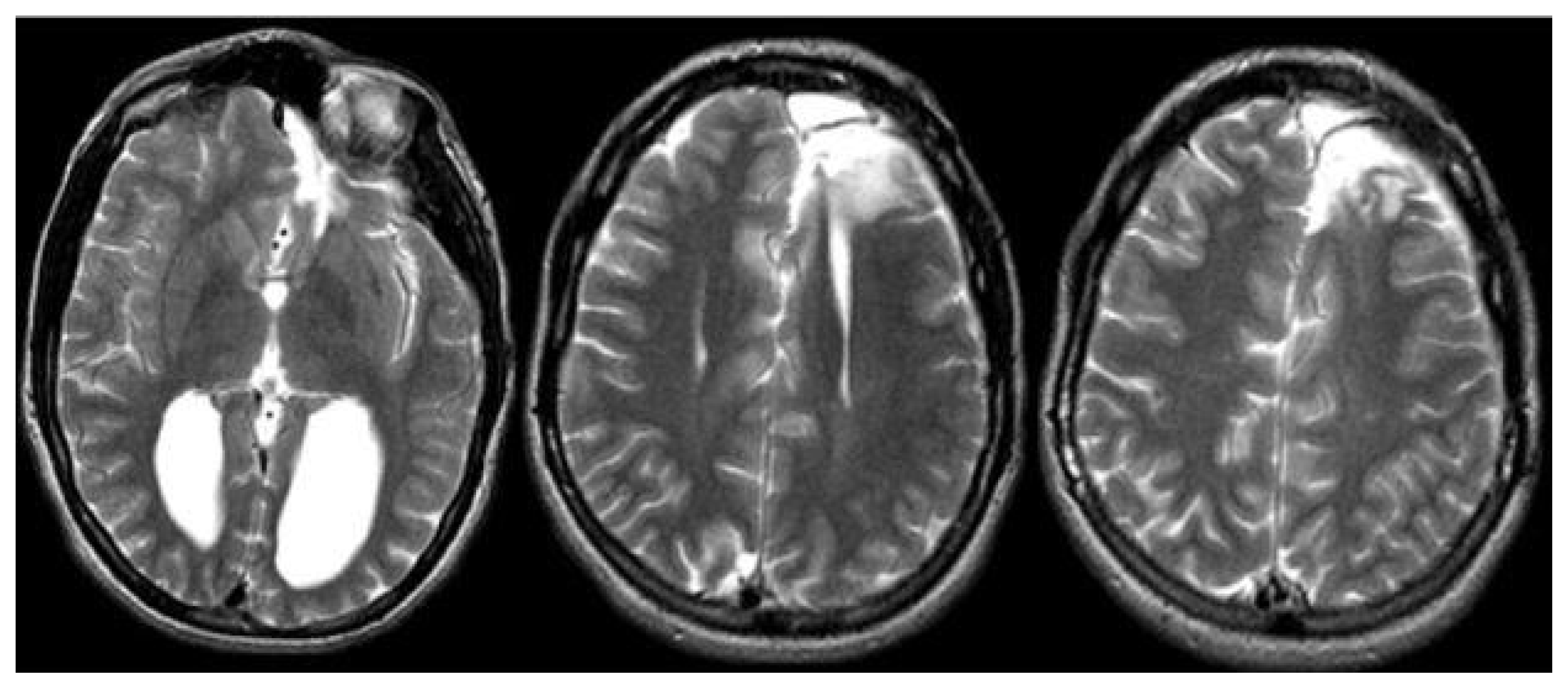
Magnetic resonance imaging confirmed complete ACC and also showed polymicrogyria within the left frontal lobe as well as a small 2 mm gray matter heterotopia within the left frontal white matter. Additionally, she had enlargement of the temporal horns and colpocephaly of the lateral ventricles (Fig. 1). Interictal scalp electroencephalography (EEG) showed left frontal spike, polyspike, and sharp and slow wave discharges, often with a broad field. Ictal scalp EEG showed bilateral attenuation of background frequencies with diffuse low voltage fast activity at seizure onset. After discussing the data at the multidisciplinary Epilepsy Surgery Conference, a decision was made to proceed with SEEG to determine if either focal resection or cortical stimulation were feasible. The SEEG implantation strategy (Fig. 2) aimed at extensive sampling of the left frontal lobe, including contacts adjacent to the anterior commissure, as well as sampling right frontal lobe, including motor area, supplemental motor area, and orbitofrontal cortex. The SEEG showed bifrontal slow waves interictally, along with high amplitude spike bursts in the left hemisphere maximal in the left dorsolateral frontal lobe. Stereo EEG showed ictal onset in the left dorsolateral frontal lobe with clear ictal spread within 100 msecs from the left to right hemisphere through the anterior commissure (Fig. 3). In addition, tractography was performed between the contacts involved in seizure onset (left frontal lobe) and the contacts where seizure spread was detected (right supplementary motor area) (Fig. 4).
Preoperative magnetic resonance imaging displaying agenesis of corpus callosum (A), foci of gray matter heterotopia on the left frontal lobe (B), colpocephaly (C), and polymicrogyria/focal cortical dysplasia on the left superior frontal gyrus (D).
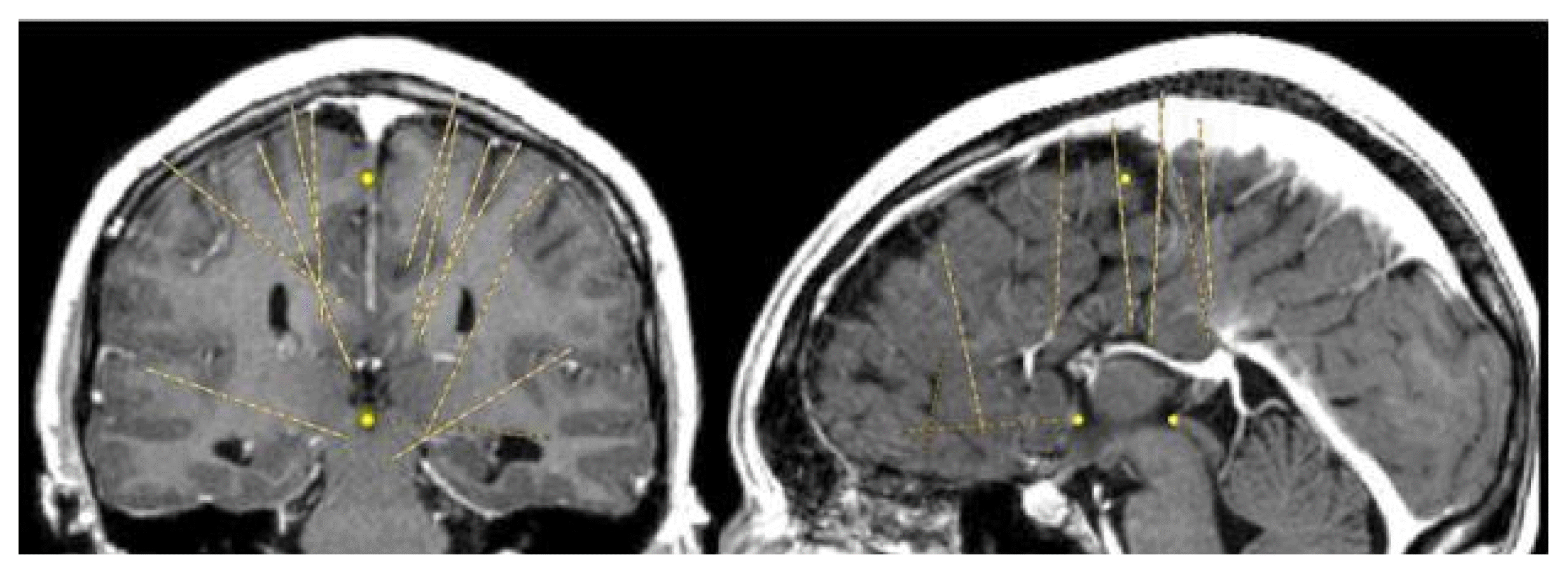
Stereoelectroencephalography plan demonstrating bilateral frontal implantation with coverage of motor area, supplemental motor area, and orbitofrontal cortex.
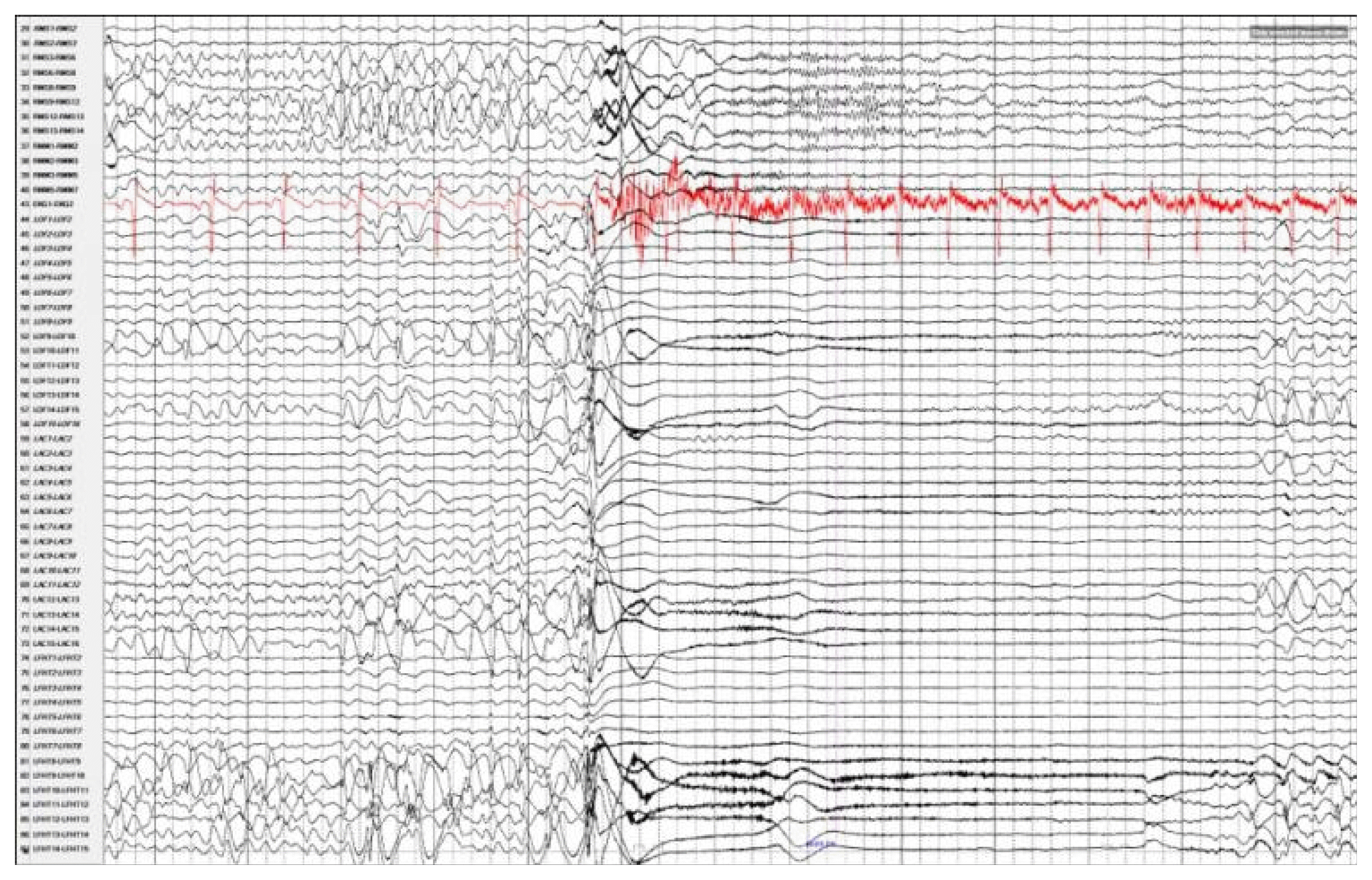
Stereoelectroencephalography demonstrating seizure spread through the anterior commissure. There is initial ictal activity seen in the left frontal heterotopia and left orbitofrontal regions, with nearly simultaneous involvement of the left anterior commissure, then rapid spread to the right frontal lobe.
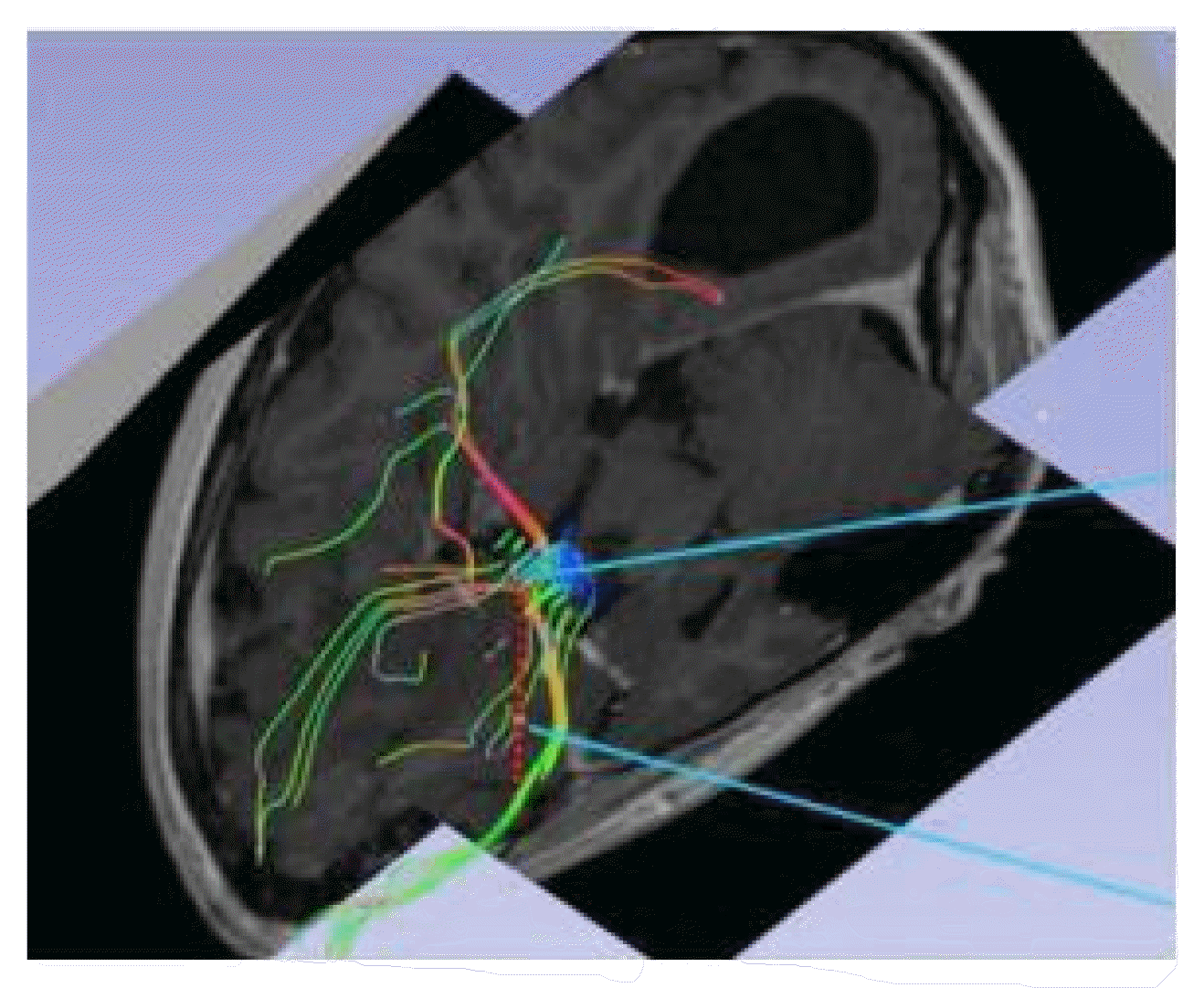
Tractography showing the fibers connecting left-sided seizure onset zones and right-sided seizure spread in the supplemental motor area.
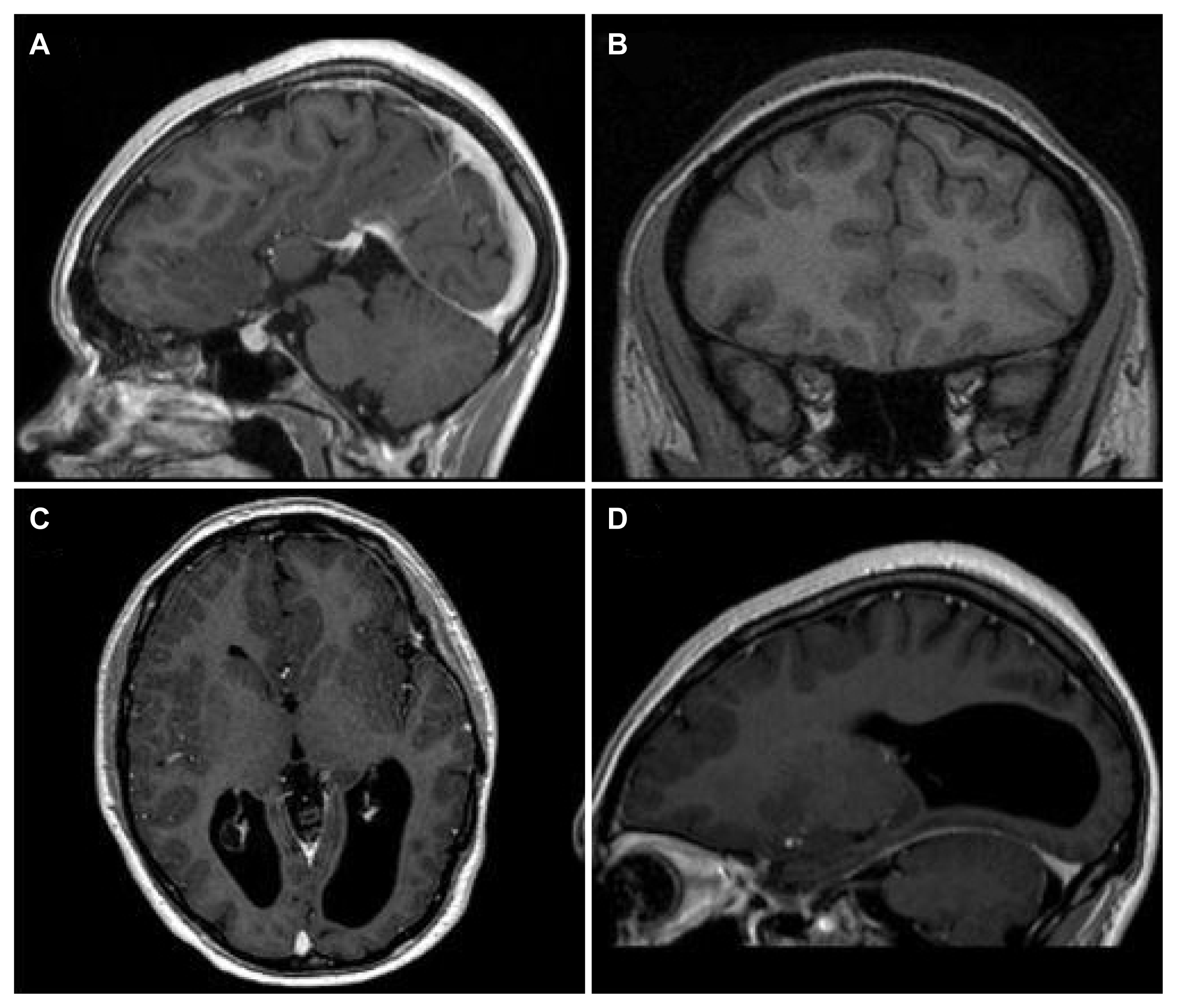
A decision was made to offer a partial left frontal lobectomy as a palliative procedure given the severity of her symptoms. This was performed, resecting gray matter involved in the SEEG seizure onset (Fig. 5). She had no motor or speech deficits post-operatively, only some mild personality changes and increased irritability, for which she was referred for cognitive behavioral therapy; these later resolved. Two years after surgery, the patient reported milder focal aware seizures. Of note, the startle-triggered focal seizures no longer caused a complex bilateral stereotyped movement; rather, she would briefly laugh and have momentary left arm and leg weakness on average 1–2 times/week. She would more gradually lower herself to the ground at times with seizures, but no longer suddenly collapsed as she did preoperatively, and did not injure herself. She has not had postoperative EEG monitoring.
Discussion
Corpus callosum and epilepsy
The corpus callosum is the largest commissure of the brain with about 190 million axons crossing the midline.14 This structure is involved in the spread of excitatory/inhibitory signals between the two hemispheres playing an important role in bilateral movements, integration of bilateral sensory and visual information, language, handedness, emotion, behavior, cognition, and memory.10,15 Two major types of structural pathologies of the corpus callosum can be associated with epilepsy: microstructural changes and developmental anomalies.16 ACC has been extensively associated with seizures, with epilepsy reported in up to two thirds of patients with complete or partial agenesis.10 However, given the often-asymptomatic nature of isolated ACC, the presence of seizures with congenital callosal abnormalities may hint at another brain pathology. In a case series of 73 patients with ACC, Nieto-Barrera et al.,11 reported 25 presenting with seizures and 24 had other associated brain abnormalities. Taylor and David,16 reported a case series of 56 patients with ACC, 57% (32) of whom had some form of epilepsy.
Anterior commissure – anatomy, connections, and role in epilepsy
The anterior commissure has been implicated in attention, visual processing, and color perception.17 However, the role of the anterior commissure in the spread of generalized seizures has not been well-investigated. One study by Miró et al.,18 showed that the anterior commissure played a contributory role in the spread of temporal lobe epilepsy in patients with hippocampal sclerosis. However, a previous study by Lieb et al.,19 suggested that the anterior commissure, along with the hippocampal commissure and parts of the corpus callosum, were noncontributory for the interhemispheric spread of mesial temporal epilepsy. This subject is obscured due to the relative lack of high-quality imaging such as tractography available in this very select group of patients. The anterior commissure may be involved in the spread of seizures in patients that undergo corpus callosotomy, but its importance in the development of generalized seizures is underscored in this case study. A case report by Barr et al.20 reported an enlarged anterior commissure in a patient with ACC which was posited to contribute to the patient’s relative lack of cognitive disability when compared to another patient with ACC who had a normal anterior commissure. This suggestion of the anterior commissure serving to compensate for the lack of interhemispheric connection in patients with ACC is supported by the EEG findings in this patient.
Other commissures
Along with the aforementioned anterior commissure and corpus callosum, other structures are responsible for seizure propagation: the posterior commissure, commissure of the fornix or the hippocampal commissure, and the habenular commissure. The role of the other, smaller commissures in generalized epilepsy is not strongly characterized, but the hippocampal commissure has been described the most extensively in relation to epilepsy. A case report by Rosenzweig et al.,21 showed seizure spread utilizing the dorsal hippocampal commissure, and suggested this commissural pattern of seizure spread to be associated with pure amnestic seizures. An early case series by Harbaugh et al.,22 showed positive results utilizing separation of the corpus callosum and underlying hippocampal commissure, but it is unknown if this additional separation confers additional seizure protection versus standard corpus callosotomy. In a more recent study, Rashid et al.,23 showed seizure reduction with low frequency stimulation of the ventral hippocampal commissure in a rat model of human temporal lobe epilepsy.
In addition, midline thalamic and subcortical nuclei have been implicated in limbic epilepsy and may play a role in both initiation and spread of seizures.24,25
Limitations
Although depth electrodes allow for deep structures to be sampled, the location and the number of electrodes that can be implanted without increasing the risks is limited. The implantation strategy is based on the hypothesis formulated with data gathered during phase I monitoring. In this case, the SEEG recording was able to demonstrate seizure propagation through the anterior commissure; however, there were no electrodes targeting the posterior commissure, hippocampal commissure, or the interthalamic adherence, and thus neuronal activity was not sampled in those structures. There is a chance the other commissures were also involved in the contralateral spread and to assert that only the anterior commissure was involved, additional electrodes would be necessary. In addition, failure to achieve complete seizure freedom suggests the possibility of an independent epileptogenic area in the right hemisphere.
Conclusion and review
In this case we present a patient with ACC and medically refractory epilepsy who underwent SEEG. Focal seizure onset was demonstrated on invasive monitoring and the patient underwent left frontal lobe resection after which less frequent and milder focal aware seizures were observed. The spread of the generalized seizures in this patient through the anterior commissure as shown on SEEG is discussed. While the connectivity of the anterior commissure has been well explored, the contribution of the anterior commissure to ictal spread has been poorly investigated and additional electrostimulation and ictal tractography studies are needed to further clarify this structure’s role in ictal spread. Patients with ACC can experience improvement using conventional resective methods, but clinicians should be aware of the existence of alternative pathways of seizure spread and possible clinical sequalae.
Notes
Conflict of Interest
The authors declare that they have no conflicts of interest.