Cerebral Salt Wasting Syndrome Associated with Status Epilepticus
Article information
Abstract
Cerebral salt wasting syndrome (CSWS) is defined as a renal loss of sodium in cerebral disorders causing hyponatremia and loss of extracellular fluid volume. Similar laboratory findings may be seen in other conditions such as syndrome of inappropriate antidiuretic hormone secretion (SIADH). A 58-year-old male visited our emergency department because of the sudden development of seizures during sleep. Magnetic resonance imaging revealed subtle high signal intensity in the right hippocampus on diffusion-weighted imaging. Ictal rhythmic discharges were observed in the concordant area. Altered metal status, polyuria and laboratory test findings including hyponatremia were compatible with CSWS. After hydration and salt replacement, his mental state and hyponatremia gradually recovered. For diagnosing CSWS, meticulous physical examinations including analysis of fluid balance are essential. CSWS should be considered in patients with hyponatremia and polyuria. Accurate diagnosis of CSWS and SIADH is crucial as the treatment plans for these two conditions are completely different.
Introduction
Cerebral salt wasting syndrome (CSWS) occurs when there is a renal loss of sodium in cerebral disorders causing hyponatremia and loss of extracellular fluid volume.1 Various disorders of the central nervous system (CNS) such as traumatic brain injury (TBI),2 meningitis, 3,4 and subarachnoid hemorrhage (SAH)5–7 are associated with CSWS, and similar laboratory findings may be seen in other conditions such as syndrome of inappropriate antidiuretic hormone secretion (SIADH).1 However, the full pathogenesis of CSWS is not completely understood. Herein, we report the case of a patient who showed CSWS following status epilepticus (SE) and discuss the pathomechanisms.
Case Report
A 58-year-old male visited our emergency department because of the sudden development of seizures during sleep. He had no previous history of seizures or a family history of epilepsy. During sleep, he developed two generalized convulsive seizures approximately 10 minutes in duration and accompanied by tongue bite, free voiding, and postictal confusion. He had retired from his job 1 year before visiting our hospital and had consumed one to two bottles of soju nearly every day during retirement thus far. He complained of general weakness after the seizures, and no other medical conditions were present.
On neurological examination, the patient was mildly drowsy but could be awakened by verbal commands. During history-taking, he often became dull and had inappropriate responses to the directions of medical personnel. No other focal neurological abnormalities were noted. Emergency laboratory tests revealed moderate leukocytosis (18,500/mm3 [normal value: 4,000–10,000/mm3]), mild elevation of liver enzyme levels (aspartate aminotransferase, 78 IU/L [normal value: 0–40]; alanine aminotransferase, 49 IU/L [normal value: 0–40]) and creatine phosphokinase level (203 IU/L [normal value: 34–145]), and a significant reduction in serum sodium (119 mEq/L [normal value: 135–145]) and serum osmolality (252 mOsm/kg [normal value: 285–295]). Other laboratory tests including blood urea nitrogen, creatinine, ionized calcium, ammonia, urine osmolality, thyroid hormones, and anti-thyroid antibodies (anti-microsomal antibodies and anti-thyroglobulin antibodies) revealed no abnormalities.
On magnetic resonance imaging, subtle high signal intensity was observed in the right hippocampus on diffusion-weighted imaging (DWI) but was not definite on T2-weighted images (Fig. 1). Electroencephalography (EEG) revealed intermittent rhythmic theta activity lasting 10–20 seconds in the right temporal area followed by slow waves, which was concordant with the DWI lesion (Fig. 2).
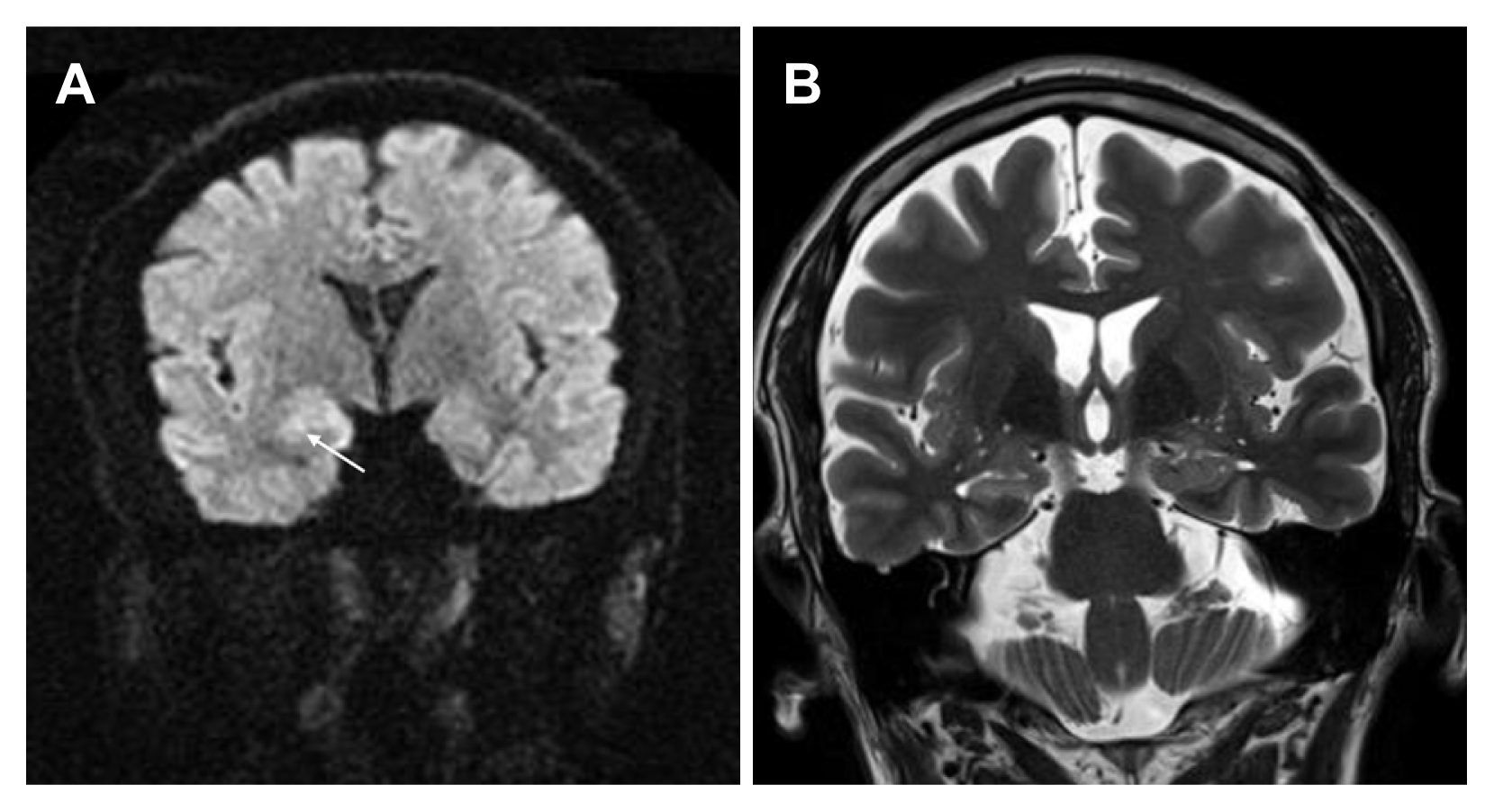
Subtle high signal intensity is observed in the right hippocampus (white arrow) on diffusion weighted image (A), which is not evident on T2-weighted image (B).
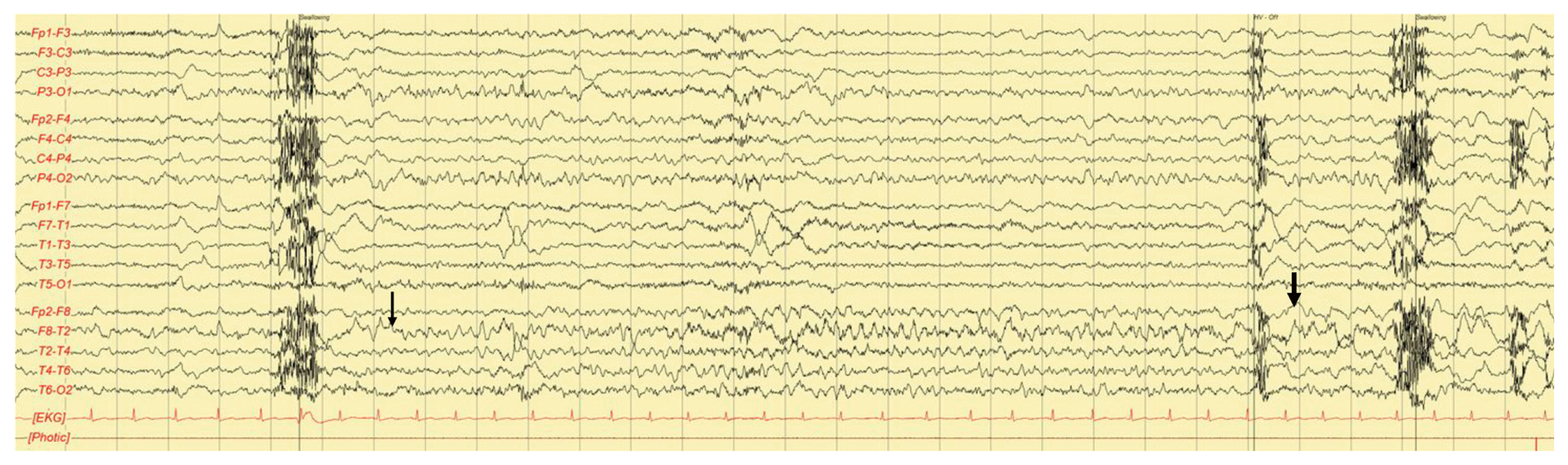
EEG shows a rhythmic theta activity (thin arrow) followed by slow waves (thick arrow) in the right temporal area. EEG, electroencephalography; EKG, electrocardiography.
On cerebrospinal fluid (CSF) examination, 3 lymphocytes/mm3 (normal value: <5/mm3) and mildly elevated protein levels (48 mg/dL [normal value: 15–40]) were observed. Other CSF tests including immunoglobulin (Ig)M/IgG antibodies against herpes simplex virus (HSV), polymerase chain reaction for HSV, paraneoplastic autoantibodies (anti-Hu, anti-Yo, anti-Ri, anti-CV2/CRMP5, anti-Ma2, and antiamphiphysin), and antibodies targeting neuronal cell-surface antigens (leucine-rich glioma inactivated-1, N-methyl-D-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and γ-aminobutyric acid type B) were all negative.
After administering intravenous fosphenytoin (loading dose: 25 mg/kg, maintenance dose: 6.5 mg/kg), the patient became alert without any recurrence of seizures. The seizure activity on EEG decreased gradually and disappeared completely on the 2 days of admission. However, the patient became confused and irritable on the 3 days of admission. Polyuria was observed from the 1 day of admission, and a negative fluid balance was also observed (intake and output: 3,750 mL and 5,900 mL on the 1 day, and 5,500 mL and 6,100 mL on the 2 days). Urine sodium and chloride levels measured on the 2 days of admission were 112 mEq/L (normal value: <20) and 125 mEq/L (normal value: <20), respectively.
Under the diagnosis of CSWS, the patient was treated with massive hydration and salt replacement (3 L of 0.9% saline per day and intermittent bolus injection of 3% saline). Serum sodium levels and osmolality gradually increased to 132 mEq/L and 280 mEq/L, respectively, on the seventh day of admission. His mental state gradually recovered, and he was transferred to a tertiary hospital as his family members wanted a second opinion regarding his seizures and mentality.
Discussion
CSWS is often complicated in patients with severe CNS disorders such as TBI, SAH, and brain tumors.1–7 Hypovolemia and hyponatremia associated with polyuria are the main clinical features; however, serum and urine osmolality may be either low or normal in CSWS.1 Serum sodium levels are low and urine sodium levels are typically high resulting from increased sodium excretion in the kidney.1,8
Similarly, hyponatremia is also present in patients with SIADH, which is common in various CNS disorders and pulmonary disorders as well.1,9 SIADH is caused by inappropriately increased secretion of antidiuretic hormone that is not suppressed by further decreases in serum osmolality. Urine output may be decreased due to the reabsorption of free water in the kidney.9 Hypovolemia is not a typical manifestation of this disorder, but low serum osmolality accompanied by high urine sodium and osmolality levels is a common laboratory finding, which is also common in CSWS.1,8,9 However, hypovolemia and polyuria, the two characteristic findings of CSWS, do not manifest in SIADH.
Accurate diagnosis of CSWS and SIADH is crucial as the treatment plans for these two conditions are completely different; fluid restriction and vasopressin may be required in SIADH, whereas treatment with isotonic or hypertonic saline is required in CSWS.1,10 To compensate for renal salt-wasting, vigorous replacement of sodium should be performed in CSWS. Additionally, to prevent osmotic demyelinating disorders such as central pontine demyelination, the maximum total change in serum sodium level should not exceed 20 mEq/L per day.8
The pathomechanisms of CSWS have yet to be fully elucidated. However, increased sympathetic activity associated with brain injury may induce increased perfusion and distend the atrial wall.1 Additionally, increased secretion of atrial natriuretic peptides and brain natriuretic peptides (BNPs) may increase angiotensin II and endothelin, which may in turn cause the disruption of the renin-angiotensin-aldosterone mechanism and the eventual natriuresis.1,8,9 Specifically, BNP may play a central role in CSWS.5 In addition, long-lasting seizures as well as convulsive or nonconvulsive SE may cause increased sympathetic activity and secretion of BNP, which may result in natriuresis.11 BNP level was not measured in our patient and this is the limitation of our study.
High signal intensity in the hippocampus shown on DWI can be associated with SE, HSV encephalitis, or autoimmune encephalitis associated with paraneoplastic syndromes or antibodies targeting neuronal cell-surface antigens.12–14 Focal temporal onset SE, hyponatremia, and high signal intensity shown on DWI are also characteristic findings of LGI1 encephalitis.15,16 To exclude these disorders from diagnosis, CSF examination should always be performed.
CSWS is often misdiagnosed as SIADH, which can be deleterious to patients. For an accurate diagnosis, meticulous physical examinations including analysis of fluid balance are essential. Moreover, CSWS should be considered in patients with hyponatremia and polyuria.
Notes
No potential conflict of interest relevant to this article was reported.