The Frequency and Precipitating Factors for Breakthrough Seizures in Children with Epilepsy
Article information
Abstract
Background and Purpose
To determine the common precipitating factors for breakthrough seizures in children with epilepsy.
Methods
This retrospective study reviewed the charts of children with epilepsy who were followed up in the pediatric neurology clinic of King Fahad Hospital in Al-Baha region, Saudi Arabia, between January 2015 and August 2022. Children between 1 to 14 years of age who had epilepsy, as per the International League Against Epilepsy definition and received anti-seizure medication with a seizure-free period of at least 2 months before breakthrough seizure episode, were included in the study.
Results
Of the 108 children included in the study, the mean age was 6.8±1.6 years, and among them (55.5%) were male. Most parents (69.5%) were unaware of the triggering factors of seizure. The majority of patients (88%) reported at least one precipitating factor for breakthrough seizures and the most common one was systemic infection associated with fever (52.8%), and then non-compliance to medications in (34.3%) of the patients. In terms of the electroencephalogram, around 84 patients (77.8%) had abnormal electroencephalogram. Finally, monotherapy was maintained in 63.9% of patients.
Conclusions
We conclude that the most common trigger for breakthrough seizure is a systemic infection associated with fever and non-compliance to anti-seizure medications. Increasing the level of awareness by different methods may help limit or even prevent seizures from occurring. Randomized controlled trials could shed light on the adjustment of anti-seizure medications temporarily by increasing the dosage or giving extra doses during the infection to avoid breakthrough seizures.
Introduction
Breakthrough seizures can have severe clinical consequences and negatively impact the patient's family and social life. It can progress to status epilepticus, which requires hospitalization.1 There is no clear definition for breakthrough seizure, but different reviewers define it as seizures that occur in epileptic patients on the optimal dosage of anti-seizure medications (ASMs) who had previously reason-able consistent control of epilepsy and then got another seizure.2,3 The International League Against Epilepsy (ILAE) considers breakthrough seizures as evidence of inadequate control and hence treatment failure after excluding poor treatment compliance and planned dose reductions.4 Despite the increased use of ASMs, long-term seizure control has remained the same.5,6 It is found that around 37% of the patients who have controlled seizures may still experience breakthrough seizures.7 Many patients with epilepsy may have breakthrough seizures despite good compliance with ASMs.8 In children, infection was the most common factor for breakthrough seizures. Other common factors that have been proposed include poor compliance with ASMs, sleep deprivation, and playing video games or watching TV.9 Epilepsy with underlying brain damage from various causes such as hypoxic ischemic injury and patient with global developmental delay can have breakthrough seizures even with adequate compliance and no obvious triggers.10
Although breakthrough seizure is common, in our country the data is scarce on children. Recognizing and avoiding such factors as an adjuvant to ASM therapy may prove beneficial. Hence, we conduct this study to identify the frequency and precipitating factors for breakthrough seizures in childhood epilepsy.
Methods
Study design
This retrospective study reviewed the charts of children with epilepsy who were followed up in the pediatric neurology clinic of King Fahad Hospital in Al-Baha region, Saudi Arabia, between January 2015 and August 2022, which is a tertiary center that caters to the healthcare needs of primarily rural and suburban people in and around this region, after the approval of the Institutional Review Board (IRB). Children between 1 to 14 years of age who had epilepsy, as per the ILAE definition and received ASM with seizure-free periods of at least 2 months before breakthrough seizure episodes were included in the study.11 Participants under one or more than 14 years of age, those without a seizure-free period of at least 2 months before a breakthrough seizure episode and patients with insufficient information were excluded.
Data collection
Data were collected from the patient's medical records, which were encoded in an excel sheet. Data included the age, gender, medical and developmental history, family history of epilepsy, socioeconomic status, epilepsy type, number and duration of the breakthrough seizure, awareness about seizure triggering factors, electroencephalogram (EEG) and neuroimaging findings, and the therapy. The type of epilepsy was classified as per ILAE.11 The selected factors precipitating seizures were based on potential triggers identified from previous studies, which include infection, non-compliance, sleep deprivation, emotional stress, using an electronic device, and constipation. The reason for non-compliance, for example, missing or disliking medicines and not accessible drugs, was collected.
Statistical analyses
Statistical analysis was performed using IBM SPSS statistics for windows, version 26 (IBM Corp., Armonk, NY, USA). All numerical variables were analyzed and described as means and standard deviations. On the other hand, categorical variables were analyzed in the form of counts and percentages. Statistical significance was set at p<0.05.
Ethical considerations
This study was approved by the Institutional Review Board of the King Fahad Hospital in the Al-Baha region, Saudi Arabia (IRB: No. 23082022/4). The study was conducted following the Declaration of Helsinki for human studies. Informed consent was waived due to the retrospective nature of the study. Confidentiality of the data was ensured for all participants.
Results
A total of 108 patients with breakthrough seizer from January 2015 to August 2022 were included in the study. Information regarding demographic characteristics, medical and developmental history, and potential seizure precipitant of these patients are described below.
Demographics, clinical, workup and management characteristics of patients
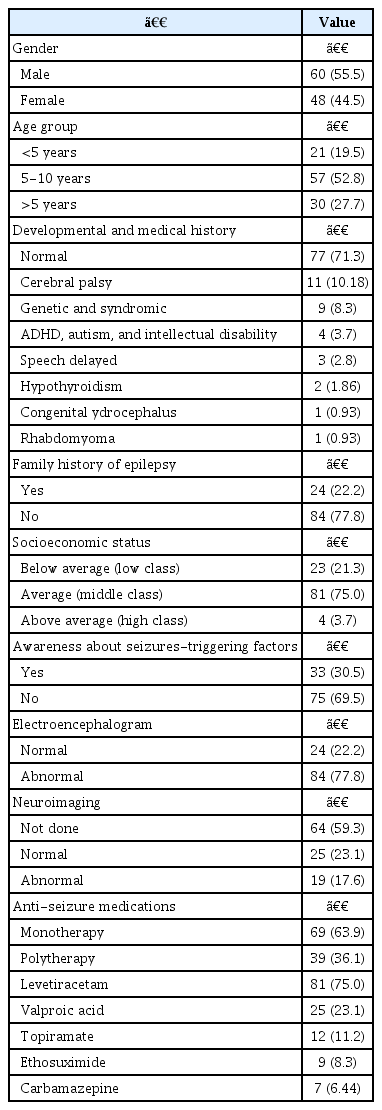
Among the 108 patients, 60 (55.5%) were male and 48 (44.5%) were female. The average age was 6.8±1.6 years, ranging from 1 to 14 years of age, and the patients aged between 5 to 10 years had the most significant burden of breakthrough seizures (57%). Additionally, 24 patients (22.2%) had a positive family history of epilepsy and around (75%) of the patients belonged to a socioeconomic status equivalent to the middle class. In approximately 69.5% of the cases, the parents and caregivers were unaware of the common precipitating factors for breakthrough seizures.
Among the cohort, 77 patients (71.3%) had normal medical and developmental history, and 11 (10.18%) had cerebral palsy. Furthermore, nine patients had genetic and syndrome disorders, while another had neurodevelopmental and behavioral disorders such as attention-deficit hyperactivity disorder and autism.
EEG findings were reported for all patients. Eighty-four patients (77.8%) had abnormal EEG. On the other hand, brain magnetic resonance imagings (MRIs) were reported in 44 (40.7%) of the patients, of which 19 had abnormal findings. In total, 69 patients (63.9%) were on monotherapy and 39 were on more than one ASM. Most patients (81.75%) were prescribed levetiracetam, followed by valproic acid in 25 patients (23.1%). Table 1 summarizes the demographics, clinical, workup and management characteristics of the patients.
Epilepsy and seizure triggering factors characteristics
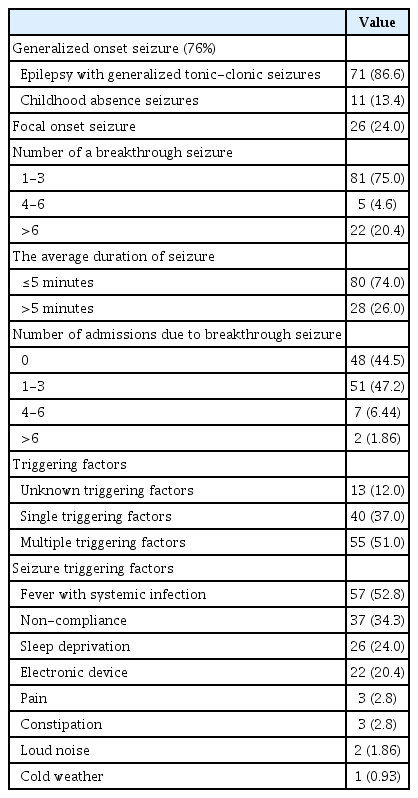
The majority of the patients, 82 (76%), had generalized onset seizure, of which 71 had epilepsy with generalized tonic-clonic seizures, 11 had childhood absence seizures, and the remaining patients had focal onset seizures. Eighty-one (75%) of the patients had one to three breakthrough seizures, while 22 (20.4%) of those patients had more than six times. Additionally, 80 patients reported duration of seizure less than or equal to 5 minutes. Finally, around (55.5%) of the patients required hospital admission due to breakthrough seizures.
Different precipitating factors for breakthrough seizures were observed in this cohort, with most patients having at least one precipitating factor (88%). Among the patients, systemic infections associated with fever, such as chest and upper respiratory tract infections, are the most commonly reported factor, accounting for (52.8%) of the cases, followed by non-compliance in (34.3%), sleep deprivation (24%) and electronic device (20.4%). Less commonly, pain, constipation, and loud noise were reported, mainly in patients with cerebral palsy. Among the reasons for non-compliance, 25 patients (67.5%) reported forgetting to take medicine as the most common cause. Table 2 summarizes the characteristics of epilepsy and seizuretriggering factors characteristics.
Discussion
Few studies have investigated the precipitating factors for breakthrough seizures in children. Therefore, our study aimed to explore the frequency and common precipitating factors for breakthrough seizure in a cohort of 108 children who were followed up at a tertiary center.
We found that the prevalence of breakthrough seizures is slightly higher in males than females, with a mean age of 6.8±1.6 years, which may be due to more adherence and better compliance to medication in female than male patients. Generally, female patients are more compliant with therapy and seek health care better compared to men.12,13 Additionally, we found that children aged between 5 to 7 years had more chances of having breakthrough seizures than other age groups, which could be due to more prone to infection or other environmental changes. Similarly, Khalid et al.14 found that 57.6% of their patients were male, with a mean age of 6.3±4.7 years. In previous studies, low socioeconomic status has been associated with an increased risk of breakthrough seizures.14,15
However, around two-thirds of our patients belonged to middle-class socioeconomic status. Our country provides free universal healthcare coverage through several government agencies, so the poverty is not the only single factor or significant cause of ill health and a barrier to accessing healthcare when needed.16 Additionally, we found that most parents and caregivers were unaware of the triggering factors of seizures, which significantly impacted the patient's and family's quality of life.
In this study, we observed that around one-fourth of the patients had a family history of epilepsy and more than three-quarters had normal developmental and medical history apart from epilepsy; this might be due to most of the epileptic patients with comorbidities following in highly specialized centers for advanced diagnostic skills, medications and equipment's, that may needed, which are not avail-able in our hospital.
Multiple studies found that structural malformation, genetic disorders, and severe brain injury were a predictor of poorly controlled epilepsy.17,18 In our study, we also found that patients with cerebral palsy or who have genetic or syndromic causes have a high rate of breakthrough seizures. In our study, most patients (76%) have generalized epilepsy, while the remaining had focal epilepsy type, consistent with the previous research results.9 This probably reflects that generalized epilepsy is the most common type of epilepsy. Moreover, we found most patients had less than three breakthrough seizures from the time of diagnosis, with three-quarters reporting duration of fewer than 5 minutes, and around one-half of the patients required hospital admission for management. However, no previous study has reported similar findings.
Several studies have documented different types of seizure triggers.9,14,19 We found different triggering factors in our patients, with most presenting with more than one factor suggesting a complex interplay of mechanisms by various factors, consistent with previous studies.9,14 Precipitant factors for seizure may differ from person to person and according to the type of seizure due to varied pathophysiology.20 In the present study, systemic infections associated with fever, such as chest and upper respiratory tract infections, have been reported as the most common triggering factor for seizures in one-half of the patients. Similarly, in a study of 80 patients, Thandapani et al.9 found that most breakthrough seizures were associated with infection. The mechanism through which infections cause seizures is still poorly understood. However, it is thought to cause increases in metabolic stress and may decrease the seizure threshold.9,20 This has essential for adjusting the medication for better seizure control. On the other hand, missing medication, emotional stress, and sleep deprivation are the most common triggering factor in adults, which is attributed to more susceptibility to infections in children than adults and underdeveloped immune systems in children.2,21
Non-compliance with medication came as the second triggering factor in around one-third of the patients. This is similar to many other studies.9,14
The most common reason for poor compliance was forgetting to take medicine which probably reflects inadequate counseling about strict adherence and maintaining a seizure diary. The second reasons were boredom and fear of the side effects of using the treatment for a long time. Sleep deprivation was the third common trigger factor reported among our patients (24%), consistent with a previous study.9 Sleep deprivation is well known to increase the incidence of interictal epileptiform discharges. Thus, it has long been used as a precipitating factor in epilepsy patients for ictal studies.22
Additionally, we found that excessive use of electronic devices was associated with breakthrough seizures in around one-fourth of our patients. This is similar to other studies that reported some cases of breakthrough seizures developed after watching TV, using flash-lights, and playing video games.9,23,24 Photic stimulation is very well known to increase the frequency of breakthrough seizures in generalized epilepsies and rarely in focal seizures. Thus, it is widely used in routine video-EEG and can contribute to diagnosing and managing patients suspected of having epilepsy.25
Constipation and pain have also rarely been reported as precipitant factors for seizures.26 However, the exact relationship is still unknown. In our study, we found that constipation and pain were associated with breakthrough seizures in most of the patients with cerebral palsy. Avorio et al.26 have described associations between constipation and increased seizure frequency among adult study participants. They observed that in the epileptic patients with drug-resistant, the seizures tended to occur or cluster mainly during constipation.26,27
Regarding the EEG, we found that around three-quarters of the patients had abnormal EEG, suggesting an association between breakthrough seizures and abnormal EEG findings. This agrees with previous studies that reported abnormal EEG findings are significant predictors of poor epilepsy control.28-30
Although neuroimaging may help evaluate patients with epilepsy, neuroimaging is not necessary or indicated for patients with generalized epilepsy, as observed in our study.31,32 In this study, brain MRI was done for around 44 patients; of them, 43% (19 patients) had abnormal findings associated with a high frequency of breakthrough seizures than the patients with normal results. Most of these were either acquired pathological or congenital findings. Similarly, Tripathi et al.33 concluded that breakthrough seizure was statistically higher among patients with abnormal neuroimaging findings.
The goal of epilepsy therapy is to help patients achieve seizure freedom without breakthrough seizures and adverse effects. Our study found that around two-thirds of the patients are on single ASM. Among them, levetiracetam is the most ASMs used. This finding has also been observed in other studies.9,14 Monotherapy has been promoted as the ideal in epilepsy treatment because of better compliance, fewer side effects, and improved seizure control compared to polytherapy. However, polytherapy also strongly impacted seizure control, especially in patients with intractable epilepsy.
This study has some limitations. First, it is a retrospective study conducted at a single center; therefore, the generalizability of the results may be limited. Second, there is no objective evidence of precipitants and it is based on the subjective perception of parents or caregivers. Further prospective studies with a larger sample and sampling from various hospitals are recommended. However, the findings of this study will provide better counseling for epilepsy patients on potential triggering factors and their avoidance.
Our data concludes that the most common trigger for breakthrough seizures is a systemic infection associated with fever, such as chest and upper respiratory tract infection, and then non-compliance to ASMs.
Increasing the level of awareness through educational programs and media alongside one another in health centers, schools, and public spaces may help limit or even prevent seizures from occurring. Randomized controlled trials could shed light on the adjustment of ASMs temporarily by increasing the dosage or giving extra doses during the infection to avoid breakthrough seizures.
Notes
The authors declare that they have no conflicts of interest.
Acknowledgments
We would like to thank and show our gratitude to the training, education center, and academic affairs at King Fahad Hospital in the Al-Baha region, Saudi Arabia.