The Significance of Insular Hypometabolism in Temporal Lobe Epilepsy in Children
Article information
Abstract
Background and Purpose:
Temporal lobe epilepsy is one of the most common forms of medically refractory epileptic syndromes. In a small percentage, temporal lobectomy fails to control the seizures in patients with epilepsy of clear temporal origin, and in some of these patients, seizures originating from the insular cortex is believed to be the cause behind the surgical failures. We retrospectively analyzed the [18F]FDG-positron emission tomography (PET) results of patients who received temporal lobe surgery for presence of insular hypometabolism, and compared the surgical outcome to verify whether insular hypometablism was related with difference in post-operative results.
Methods:
13 patients were enrolled, and clinical variables, post-operative pathology, magnetic resonance imaging and PET results were analyzed for possible differences between the patients with or without insular hypometabolism.
Results:
7 patients showed insular hypometabolism, while 6 patients were clear of insular lesion on PET exam. 8 patients received anterior temporal lobectomy with amygdalohippocampectomy (AH), 2 patients received radical temporal lobectomy with AH, and 3 patients received insular cortisectomy. Post-operative results were favorable in 8 patients and unfavorable in 5, with unfavorable outcomes in all recipients of insular cortisectomy. Presence of insular hypometabolism did not have any significant relationship with the post-surgical outcome (p=0.266), but its trend showed a tendency towards favorable outcome if insular hypometabolism was not present.
Conclusions:
Presence of insular hypometabolism in [18F]FDG-PET analysis was not significantly correlated with the post-operative outcome, and recipients of insular cortisectomy among our patients with insular hypometabolism resulted in poor surgical outcome. However, the outcome trend showed a tendency towards better surgical outcome with absence of hypometabolic lesion in the ipsilateral insular cortex. Further studies employing a larger patient group is needed.
Introduction
Epilepsy is one of the most prevalent chronic neurological diseases of childhood, and if left uncontrolled, results in permanent developmental and physical debilitation. Most epileptic seizures are rendered inactive by proper use of anti-epileptic medications, but in 30–40% of patients, such medications are inadequate in controlling the repeated bouts of seizures, and this rate has remained almost constant despite recent development of many novel therapeutic drugs.1,2 Hence, surgical intervention has gained increasing prominence in these patients.
Surgical treatment of refractory epilepsy has been an option for more than 100 years, but recent advances in neuroimaging techniques have greatly increased the efficacy, as well as safety of surgical ablation of refractory epilepsy, and we have experienced exponential growth in epilepsy surgery during the last two decades.2 Certain types of epilepsy are especially prone to good surgical outcome, as to be dubbed “surgically remediable syndromes”. These include the mesial temporal lobe epilepsy, which may be the most common form of such epilepsies, and neocortical epilepsies caused by discrete, readily resectable foci.3,4 When surgical intervention is performed promptly enough in such cases, resulting seizure ablation provides valuable opportunities for epileptic children to catch up on physical and psychological development.5 However, even though results of temporal lobe resections in pediatric temporal lobe epilepsy are excellent, with seizure freedom achieved in up to 63.3% of patients,6 a subset remains where resection of temporal lobe was not effective, although all pre-surgical evaluations pointed to ipsilateral temporal lobe origin of seizures. In some of these patients, seizures originating from the insular cortex are believed to be the reason behind the surgical failures.7,8
The semiology of insular seizure is described as being similar to seizures of temporal or frontal lobe origin.8 Coupled with the fact that surgery of the insular is not a generally accepted treatment option in many institutions due to its proximity to the language cortex, difficulty of surgical approach and abundance of hidden, essential blood vessels,9 proper selection of candidates for insular resection is difficult but paramount for better successes in epilepsy surgery. This selection, however, needs to be done with great caution due to higher risk that insular surgery entails in comparison to temporal lobe surgery.
Long-term intracranial electrocorticographic (EcoG) recording is useful for delineating patients with temporal lobe epilepsy, but it has only limited value in analyzing ictal discharges originating from such deep cortical structures as insula. Stereo-electroencephalopgraphic (SEEG) recording is useful in selecting patients with seizures of insular origin,8 but the invasive nature of this tool forbids its routine application in all temporal lobe epilepsy patients. Hence, we have focused on determining the usefulness of fluorine-18 fluorodeoxyglucose positron emission tomography ([18F]FDG-PET) studies in selecting patients in need of further invasive insular monitoring through utilization of post-surgical results after temporal lobe surgery, by verifying whether patients with insular hypometabolism present with poorer post-surgical outcome.
Methods
Patient selection
All patients who received temporal lobe surgery in pediatric neurology clinic of Severance Children’s Hospital from January 2007 to January 2009 were selected and retrospectively analyzed. Patients were excluded from the cohort if the temporal lobe surgeries were performed due to trauma, overt tumors, or if concomitant surgery of extratemporal area was performed. In all cases, the patients suffered from medically refractory epilepsy with seizures determined to originate from temporal neocortex or mesiotemporal areas, and were using at least two anti-epileptic drugs (AED) at the time of surgery. Semiologies of the seizures were defined as the clinical feature of the most prevalent and debilitating ictal event that the patient possesses, with its character determined by clinical and electroencephalographic (EEG) observation during video EEG monitoring.
All patients underwent similar pre-surgical evaluation including medical, neurological and neuropsychiatric examinations, long-term video EEG, brain magnetic resonance imaging (MRI), [18F]FDG-PET, ictal perfusion 99mTc-ethyl cysteinate dimer (ECD) single photon emission computed tomography (SPECT), and subtraction ictal SPECT coregistered to MRI (SISCOM). Patients subsequently received long-term intracranial recording by EcoG, before final determination of the extent of temporal lobe resection.
Outcome was assessed according to Engel’s classification of post-surgical outcome (Table 1).10 Engel class I was considered as favorable post-surgical outcome, and all other classes were deemed unfavorable.
Protocol for EEG acquisition and surgery
All patients received long-term EEG monitoring in a dedicated electroencephalographic monitoring unit (EMU). The tracing was obtained in accordance to international 10–20 system. In patients whom seizures were decided to originate from the temporal lobe, surgical feasibility was discussed in a board meeting attended by specialists in child neurology, neurosurgery, diagnostic neuroradiologist, and child psychologist. Epileptogenic lesion was delineated primarily by utilization of the EEG result and concomitant data acquired from neuroimaging studies. If surgery was deemed possible, the patient underwent a two-stage operation consisting of initial intracranial EcoG recording lasting a minimum of four days, with the extent of cortical electrode placement determined by the previous long-term video EEG, followed by temporal lobe resection proper, with its extent determined by the analysis of the EcoG result.
Protocol for MRI acquisition
Standard MRI was performed with conventional spin-echo T1-weighted sagittal, T2-weighted axial, flair axial, oblique coronal, and flair oblique coronal sequences, as well as with ultrafast gradient echo (TFE) T1-weighted 3D coronal sequences. Philips MRI Achieva 3.0T Release 2.5.3.3. (USA) was used to acquire the seizure-specialized sequences termed seizure phase 1 images, in accordance to the protocol described in Table 2. 3D T1-weighted TFE magnetization-prepared rapid acquisition with gradient-echo sequence and both oblique coronal sequences with 3 mm-thick-sections were obtained in the oblique coronal plane of the temporal lobes perpendicular to the long axis of the hippocampus.
Protocol for [18F]FDG-PET acquisition
PET images were acquired using GE ADAVANCE PET Scanner (GE, Milwaukee, WI, USA) in 3D mode. The transaxial resolution of the system was 5.2 mm FWHM (full-width-half maximum) at the center of FOV (field of view). Approximately 5 mCi of [18F]FDG was injected intravenously after 6hrs of fasting, and the patients were either sedated if uncooperative, or if cooperative, asked to lie still with their eyes closed in a quiet, dimly lit room during the injection and the following 40 minutes. The emission scan started at 40 minutes after the injection for 15 minutes, and eight minutes transmission scan was subsequently acquired for the purpose of attention correction. EEG was performed during the FDG uptake using portable EEG with international 10–20 system, and its tracing was later examined for presence of possible ictal discharges during the acquisition of the images. The insular cortex in PET was located by searching for the level of image where caudate, thalamus and bilateral temporal lobes are visible. Presence of interictal insular hypometabolism was determined by visual analysis of the insular cortex by qualified nuclear radiologist and pediatric neurologist specializing in childhood epilepsy.
Statistics
Patients were divided into PET positive group and insular PET negative group according to the presence of insular hypometabolism in respective [18F]FDG-PET images. Possible clinical confounding variables such as sex, side of epileptic surgery, age at operation, duration of treatment before surgery, frequency of seizures, number of AED used at the time of surgery, and follow-up duration after surgery between the two groups were analyzed for statistically significant differences. Finally, post-operative clinical outcome was compared between the two groups for confirmation of the impact insular hypometabolism has on the surgical outcome.
In analyzing the relationship between the insular hypometabolism and surgical outcome, Fischer’s exact test was used. For determination of differences in variables between the insular hypometabolic and non-hypometabolic group, Mann-Whitney test was employed for continuous variables and Fischer’s exact test for non-continuous variables. A p-value of less than 0.05 was considered statistically significant. SPSS statistical package, version 14.0 (SPSS Inc. Chicago, IL, USA) was used for statistical analysis.
Results
Clinical data
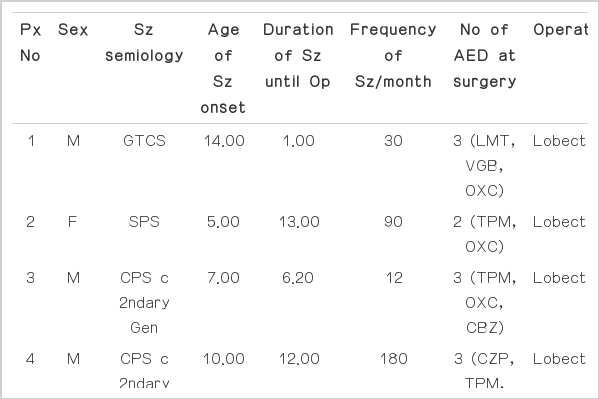
Clinical data of the cohort is summarized in Table 3. A total of 13 patients (10 males, 3 females) were enrolled in the study. Mean age of seizure onset was 6.74 years (±3.63), and the mean duration of seizure until operation was 6.83 years (±4.70). Seizure semiology consisted of complex partial seizures in 6 cases, complex partial seizure with secondary generalization in 4 cases, simple partial seizure in 1 case, generalized tonic clonic seizure in 1 case, and epilepsia partialis continua in 1 case. Patients were using an average of 2.92 (±0.95) AEDs at the time of surgery. Mean age at surgery was 13.55 years (±5.75), and the patients were followed up for a mean period of 1.83 years (±0.72).
All patients received temporal lobectomy. Lesions were right-sided in 5 cases and left-sided in 8 cases. Eight patients received anterior temporal lobectomy (ATL) with amygdalohippocampectomy (AH), 2 patients received radical temporal lobectomy (TL) with AH, 1 patient received ATL and AH with tailored cortisectomy of the insular cortex, 1 patient received TL and tailored insular cortisectomy, and 1 patient received TL and AH with tailored insular cortisectomy. Pathological analysis of resected cortex revealed normal pathology in 8 cases, microdysgenesis in 3 cases and cortical dysplasia in 2 cases. Pathological analysis of the hippocampus, in cases where hippocampectomy was performed (n=12), revealed hippocampal sclerosis in most cases (n=10), and normal pathology in the rest (n=2). Post-operative outcome after a mean duration of 1.83 years showed successful seizure ablation in 8 patients (Engel class Ia). Surgical failures were observed in 5 cases, specifically, Engel class IIa in 1 patient, IIb in 3 patients and IIIa in 1 patient. No patient showed worsening of seizures post-operatively.
MRI Data
Lesion in temporal neocortex was visible in 7 cases, while mesiotemporal lesion was visible in 3 cases. Hippocampal sclerosis was observed in 8 cases. One patient showed overt signal change in the insular cortex, and MRI result was unremarkable in 1 patient.
[18F]FDG-PET Data
[18F]FDG-PET analysis revealed interictal temporal hypometabolism in 9 patients and mesiotemporal hypometabolism in 7 patients. Interictal hypometabolism in the basal ganglia was noted in 1 patient, while 1 patient showed generalized decrease in [18F]FDG uptake over the whole hemispheres. Interictal insular hypometabolism was noted in 7 cases while 6 patients showed normal insular uptake.
Statistics
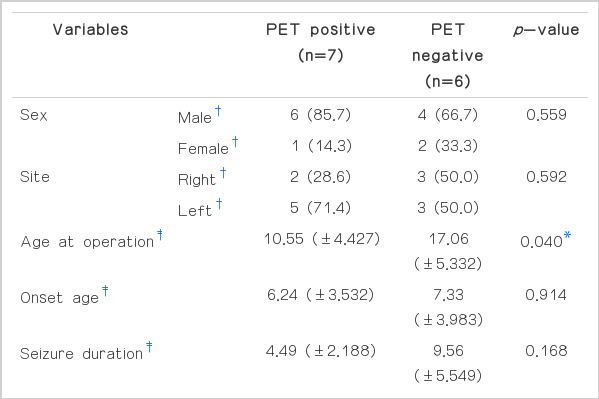
Table 4 depicts the statistical values and relevance of clinical variables in relation to the presence of insular hypometabolism. Fisher’s exact test for sex and the site of seizure focus did not show any statistical significance between the PET positive and PET negative group. Mann-Whitney test for the age of operation, age at seizure onset, duration of seizure until surgery, seizure frequency, number of seizure medications, and the duration of post-operative follow-up did not show any statistical differences between the PET positive and negative group in most incidences. However, statistically significant difference was present for the age at operation, and this was the only statistically relevant difference between the two groups. Sample sizes for post-operative pathology results, seizure characteristics, MRI, and [18F]FDG-PET results were too small to employ any meaningful statistical analysis.
Odd ratio for the risk of unfavorable surgical outcome in relation to insular hypometabolism was 6.667, with a 95% confidence interval of 0.487–91.331, and the p value of statistical significance between the presence of PET lesion and post-surgical outcome by Fisher’s exact test was 0.266 (Table 5). Although the relationship between the presence of insular hypometabolism on PET images and post-surgical outcomes was not statistically significant, the trend pointed towards better surgical outcome for PET negative groups, with 83.3% of the patients showing favorable outcome if insular hypometabolism was absent (Table 5, Fig. 1).
Clinical outcomes of recipients of insular cortisectomy
Three patients, designated patients 3, 9 and 12 in Table 3, received insular cortisectomy along with temporal lobectomy. All of them presented with insular hypometabolism in [18F]FDG-PET. MRI showed temporal lesions in 2 patients and mesiotemporal sclerosis with overt insular lesion in one. The mean age of seizure onset in this group was 3.72 years and the mean duration of seizure until operation was 4.43 years, all of which were lower than the mean values of the total cohort group. Two patients presented with complex partial seizures with secondary generalization, and one presented with epilepsia partialis continua. They were taking an average of 3.67 medications at the time of surgery, which was higher than the total average of 2.92 medications. Patients 3 received ATL and AH along with IC, while patient 9 received TL and IC, and patient 12 received TL and AH. Post-operative pathologies revealed microdysgenesis in 2 patients and cortical dysplasia in 1 patient, while hippocampal sclerosis was noted in 1 patient. Post-operative results were poor in all recipients of insular cortisectomy, with Engel class II in 2 patients and class III in 1 patient.
Disscussion
Surgical treatment of refractory epilepsy has gained increasing prominence in recent decades. Advancements in non-invasive diagnostic modalities such as higher resolution MRI, PET scans and SISCOM have permitted more accurate localization of epileptic lesions and better pre-operative planning, resulting in improved surgical outcomes with less post-operative morbidities.11 This has resulted in routine performance of anteromesial temporal lobe resections in circumstances when epilepsy remains refractory to multiple AEDs, with high attainment of seizure freedom.12 Kwan and Brodie have observed that even with the introduction of newer AEDs since the early 1990s, the proportion of patients suffering from intractable epilepsy have stayed very much constant. In fact, it is now becoming clear that new AEDs have struck little impact on attaining seizure freedom in patients suffering from drug-resistant epilepsy.1 When these points are taken into consideration, it becomes clear that early surgical intervention is beneficiary, if not critical, in obtaining seizure freedom if more than two appropriate AEDs fail to control the seizures.13
Early seizure freedom is especially vital in pediatric patients, since repeated bouts of seizure attacks can have devastating impact on a child’s neurological development at a time when its loss would be ultimately unrecoverable. However, there exists a small subset of patients in whom temporal lobectomy provided little benefit, even though pre-surgical long-term EEG and imaging modalities such as MRI and PET pointed to the ipsilateral origin of temporal lobe epilepsy.
In early 1950s, Guillaume and Mazars were the first to point out that seizures originating from the insular cortex might be the reason behind these surgical failures, and stated that seizures originating from the insular cortex resembled seizures originating from the temporal lobe to such a degree that confusion on the origin of the epileptic discharge might result in erroneous resection of the brain.14,15 This hypothesis was later repeated by Penfield and Jasper,16 but the impact of insular cortex on the post-surgical outcome remained much debated.
Some later researches by other groups stated that insular involvement in mesiotemporal lobe epilepsies could explain the late persistence of disabling seizures in up to 30% of patients after anterior temporal lobe lobectomy,17–19 and this resulted in a brief surge of anterior temporal lobectomy with associated insulectomy. However, a large-scale analysis of surgical outcome among temporal lobectomy with associated insulectomy was performed from 1941 to 1962 by Silfvenius et al, and the result was compared with matching control group of usual temporal lobectomy.19 Surprisingly, no significant difference in the surgical outcome was observed among these 2 groups, and the insulectomy group presented with a much higher morbidity after the procedure. This led to abandoning of the insulectomy as one of the options for TLE patients in many institutions, and the question of insular involvement in TLE was not investigated further.
Insular surgery regained its spotlight in the 21st century when Isnard and colleagues demonstrated that seizures spreading to the ipsilateral insular cortex correlated with favorable surgical outcome, whereas seizures originating from insular itself did not benefit from conventional temporal lobe surgery.7 This article also pointed out the fact that in aforementioned Silfvenius’ research, higher percentages of patients benefited from insular surgery if it was performed after temporal lobectomy failure as opposed to patients who did not receive follow-through surgery, and suggested in concordance with his research that insular surgery is necessary if seizures originated from the insular cortex itself.
Differentiation of TLE with epilepsy of insular origin is, however, extremely difficult. Symptoms induced by insular stimulation are similar to the symptoms observed during temporal lobe seizures,16,17 and clinical observations of seizures with known insular lesion described its semiology as being similar to temporal7 and frontal20 lobe seizures. This is quite forseeable, since insular lobe and temporo-limbic structures such as olfactory cortex, cingular cortex, entorhinal cortex, amygdala, and hippocampus share dense neural connections with each other. Initial sequential occurence of laryngeal discomfort with thoracic oppression or dyspnea, unpleasant paresthesiae, or warmth sensation focused on the perioral region or extended to a large somatic territory, and dysarthric, dysphonic speech before the onset of somatomotor symptoms raises the possibility that the seizure originated from the insular cortex,8 but selection of candidates for insular cortisectomy based simply on clinical semiology remains too risky.
The most confirmative method would be to record the actual ictal discharges originating from the insula. Wieser employed SEEG recording in TLE patients to verify the route of ictal electrographic propagation and found that epileptic discharges evolving from the temporal lobe takes preferential spread from amygdale and hippocampus towards orbitofrontal cortex, which explained the high rate of hippocampal sclerosis in chronic TLE patients.21 Isnard et al employed the same SEEG technique to verify the route of ictal discharge in insular seizures, and found that discharges from TLE frequently and rapidly spreads to the insular regions by mostly following one of two constant pathways - namely, through hippocampal relay and through external temporal neocortex.7 This propagative pattern makes the differentiation between temporal and insular epilepsies difficult, as use of simple intracranial ECoG alone would not permit the differentiation of seizures of insular origin from primarily temporal lobe seizures. Since patients suffering from secondary insular epilepsy can benefit from simple ATL, and because seizures originating primarily from insular cortex are responsible for surgical failures, it is imperative for clinicians to be able to differentiate TLE patients from insular epilepsy. Simple ECoG does not provide adequate information in this regard. Invasive SEEG monitoring is the only method to confirm seizures originating from the insular cortex, but its invasiveness makes it an unattractive option for routine application.
Imaging analysis is non-invasive, and unlike EEG-based methods, they are not dependent on electrical activity of the brain. They are also performed as part of the routine pre-surgical evaluations in all patients undergoing epilepsy surgery in our institution. [18F]FDG-PET, in particular, is an important tool in delineating the ictal onset zone and in understanding the neurobiology and functional alterations caused by various forms of epilepsy. The glucose analogue tracer, [18F]FDG, is and indirect marker of neuronal activity. It allows the quantification of cerebral glucose metabolism, and interictal [18F] FDG-PET has established its role in non-invasive localization of epileptogenic foci. It also reflects the dynamic seizure-related changes in cerebral cellular functions. Interictal epileptogenic foci tend to appear as zone of hypometabolism on [18F]FDG-PET. In TLE, comparison of hypometabolism with contralateral side has been shown to be useful for localization of the epileptogenic zone.22 Numerous studies employing large number of patients have reported a sensitivity of 70–85% for [18F]FDG-PET in TLE.23–25 [18F]FDG-PET analysis is especially known to be useful in delineating the epileptic zone in MRI-negative, PET-positive TLE.23 Therefore, it can be inferred that this modality would also prove to be useful in detecting lesions proximal to the temporal lobe, such as the insula.
Our analysis, however, shows that the presence of insular hypometabolism in [18F]FDG-PET does not have any impact on the post-surgical outcome. Patients with insular hypometabolism did not differ significantly with non-hypometabolic group in regard to their clinical characteristics such as onset age, sex, number of AED used, or seizure duration, so this result does not seem to be the result of an obscure and inherent confounding variable residing within the study population. Both patient groups differed significantly only by the age at surgery, with the insular hypometabolic group receiving surgery at an earlier age. This could be considered as being caused by the non-hypometabolic group presenting with less severe symptoms, which caused delay in reaching the decision to take surgery until a later age, although the duration of seizures between the two groups was not significant. In addition to the statistical result, 3 recipients of insular cortisectomy, all of whom showed significant insular hypometabolism in PET images, ultimately presented with poor post-surgical outcomes. This may be caused by the presence of obscure dual pathology or diaschisis in other cortical areas that were not detectable in our pre-surgical evaluations. These 3 patients tended to present with earlier onset of seizures with shorter time interval to surgery, and took more anti-epileptic drugs than the mean value of cohorts, but since the absolute number of the insular cortisectomy was too small to glean any significant patterns, their clinical characteristics seems to be unhelpful until more patients are enrolled. However, their poor response to surgery is supportive of the result of our statistical analysis.
Although the statistical significance was lacking, it is noteworthy to observe that the trend shows a general tendency towards better surgical outcome in patients without insular hypometabolism. Therefore, it may be possible that with a larger number of cohort, the final result might be different. But at present, our result is in concordance with previously published report by Bouilleret et al, who stated that the degree of hypometabolism in the ipsilateral insula by FDG-PET was not related to the post-operative outcome.26 This result is understandable, since the densely interconnected neural network present in the insular cortex allows for rapid spread of ictal discharges, and this leads to an overestimation of the size of the seizure onset zone. Such wide involvement of the epileptic discharges results in wider areas of interictal hypometabolism in FDG-PET, leading to difficulties in pinpointing the area of the origin of ictal discharges. Insular resection has therapeutic meaning in only patients with primary insular epilepsy, so FDG-PET analysis seems to have limited values so far in replacing long-term SEEG in selecting patients suitable for insular surgery, but further studies are needed to verify this point.
Our study is limited in that the sample size was too small to warrant high statistical meaning to its results. The sample size was too small for analysis of certain variables such as MRI and PET results, or post-operative pathological findings. It does, however, show some promising data, such as its tendency towards better surgical outcome among PET negative group, and warrants further study in the near future with employment of a larger cohort. Also, the study can benefit from division of the cohort into more specialized subgroups, such as groups dedicated specifically to insular cortisectomy patients, and analyzing the PET findings in relation to their post-operative outcome. This will provide more precise insight into the role insular hypometabolism plays on the need for insular surgery, but insular cortisectomy is still a rare and carefully applied procedure in our institution-in fact, it is performed very sparingly in almost all institutions the world over-and the absolute size of the sample is still too small for this method. Gradual accumulation of the insular surgery data, however, is on the way, and we expect to be able to perform a more elaborate study in the coming future.