Methods
1. Subjects
A retrospective study was performed by examining in the clinical database of the Pediatric Department of Sunlin Hospital, Pohang, Korea, the records of patients seen between January 1, 2006, and December 31, 2009. The subjects were children suffering from convulsions with fever or children who had convulsions with diarrhea, regardless of fever. This study excluded patients who presented with seizures from the following causes: trauma, CNS infection, hypoglycemia, and electrolyte imbalance. Patients who had seizures while taking anticonvulsants or patients with seizures showing electroencephalography (EEG) abnormalities, in any follow-up examination for at least 6-months after admission, were also excluded. A total of 706 children who met the inclusion criteria were investigated.
A fever was defined as an ear drum temperature of 38.0°C or above. Convulsions with fever were defined by the guardians’ testimonies, according to the following: 1) seizures attack after fever occurrence or 2) fever occurrence within 24 h after convulsions, in cases in which the body temperature at admission was 38.0°C or below. Diarrhea was defined as loose stools occurring three or more times per day. Convulsions with AGE were defined as diarrhea occurring for three days or more, before or after convulsions, and regardless of fever. It excluded patients with positive stool cultures for bacterial pathogens or those with suspected symptoms of bacterial infections, such as severe dehydration, severe abdominal pain, or bloody diarrhea.
2. Methods
Routine complete blood cell counts, electrolyte panels, blood glucose levels, and urinalyses were conducted in order to determine the cause of the fever. The patient’s past history related to febrile convulsions, fever duration before the seizure, convulsion duration, family history, and the presence of delayed growth development were reviewed, based on the guardians’ testimonies. Sex and age at the time of the convulsions, total fever duration, and the presence of complex convulsions were investigated through the clinical database.
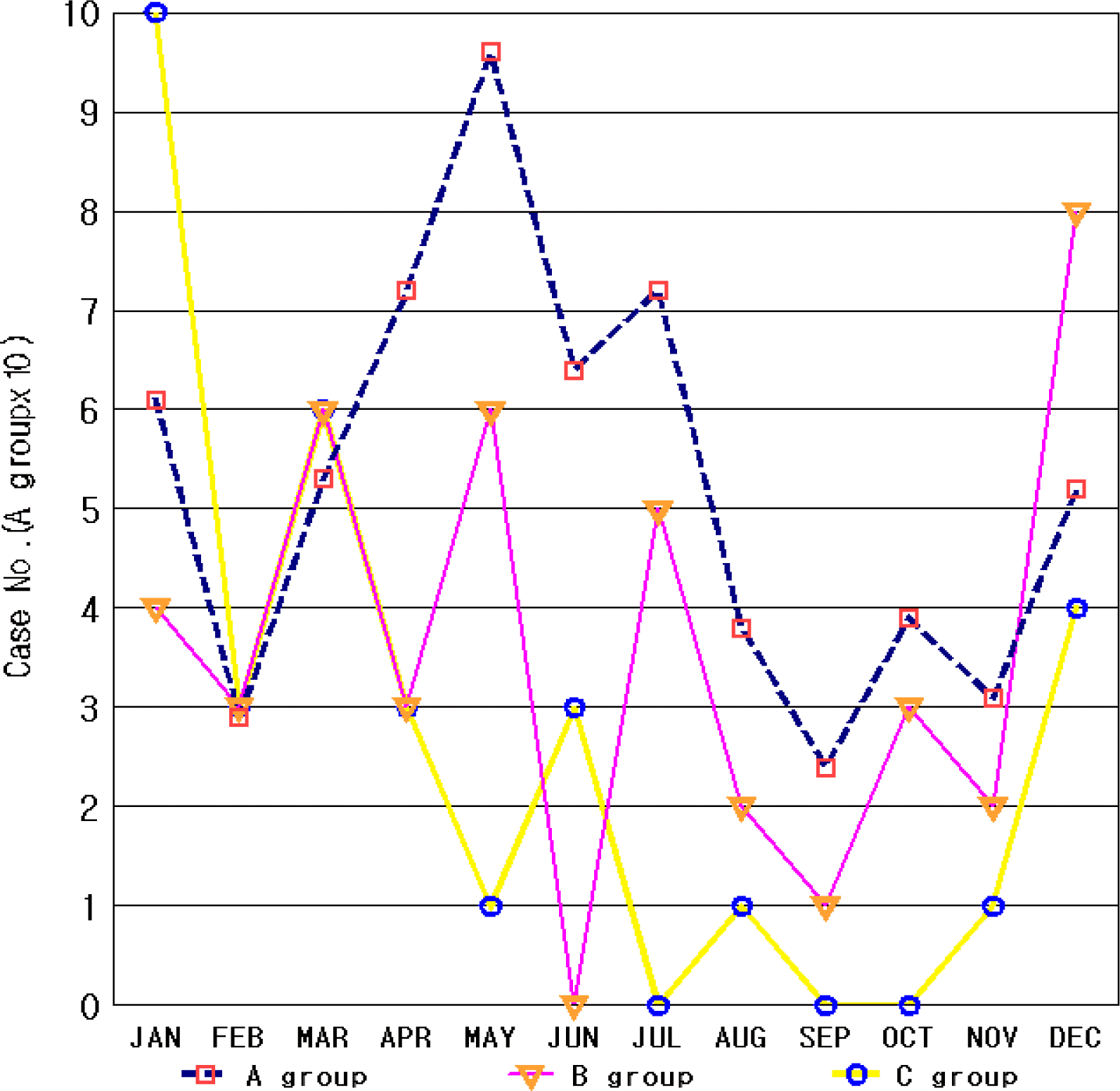
For patients who were less than 12 months old or over 6 years, or for those with suspicious CNS infections or complex febrile convulsions, Magnetic Resonance Imaging (MRI), lumbar puncture tests, and EEG were recommended. The presence of the rotavirus antigen was confirmed using the BioLine immunochromatography (Standard Diagnostics, Inc., Yongin, Korea). After analyzing the age and sex distribution, the past history of febrile convulsions, the degree of fever, the fever duration and its origin, as well as the MRI, EEG, and CSF test results in all 706 patients, the convulsion cases were classified into the following three groups: 1) group A, consisting of patients whose fever origin was not associated with AGE or patients who had no gastroenteritis symptoms; 2) group B, consisting of patients in whom gastroenteritis symptoms of non-rotavirus gastroenteritis existed, regardless of fever; and 3) group C, consisting of patients who had rotavirus gastroenteritis. The month of hospital admission, the distribution trends, and the clinical characteristics of these groups were analyzed and compared.
3. Statistics
The SPSS software version 12.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses, and the Chi-square and ANOVA tests were conducted. White Blood Cells (WBC) counts, C-Reactive Proteins (CRP) levels, and days of hospitalization were investigated for correlations. The p-value less than 0.05 (p < 0.05) was defined as statistically significant.
Discussion
Febrile convulsions are common convulsive disorders that occur in 2–5% of children aged 5 years and under, who experience at least one or more episodes of seizures. The prognosis is good if the provoking cause is removed [
7–
9]. However, in afebrile cases, it is necessary firstly to rule out several convulsive conditions, such as CNS infections, electrolyte and metabolism disorders, and organic brain diseases, and then to evaluate the prognosis. Salmi
et al. reported that rotavirus gastroenteritis can be associated with neurologic disorders [
10]. Similarly, Morooka reported that mild AGE might cause afebrile convulsions [
1]. Convulsions associated with mild AGE (CwG) have been reported mostly in Northeast Asian countries, such as Japan [
11–
13], Hong Kong [
14], and Taiwan [
15,
16], and recently, in Europe [
17] and in the United States [
18]. Thus, AGE seems a common cause of symptomatic seizures in children below age 3 [
16].
The most common causative agent for convulsions in children younger than 5 is rotavirus. Other causative agents are small round-structured viruses (SRSV), such as the Norwalk-like virus, astrovirus, calicivirus, and adenovirus [
19]. These viruses are known to produce convulsions [
12]. After rotavirus, the Norwalk virus is the second most common causative agent for children seizures in Korea [
20]. Lee and Ong observed three groups of patients with febrile convulsions, convulsions with mild AGE, and idiopathic convulsions for 5 years [
21]. Idiopathic convulsions were observed in 1.6%, 5.7% and 65.7%, respectively, in each group, and the prognosis of CwG was favorable. Verrotti
et al. suggested that the concept of CwG was necessary, and anticonvulsants or other diagnostic tests were unnecessary, a situation similar to febrile convulsions [
2].
In Korea, Koh
et al. described the clinical characteristics of nine afebrile convulsion cases associated with AGE caused by the rotavirus [
22]. Cho
et al. reported that benign convulsions with gastroenteritis was the third most common cause of convulsions in children aged from 6 months to 5 years after febrile convulsions and epilepsy [
23]. Kang
et al. reported that the frequency of neurologic abnormalities in children with diarrhea was up to 9%. Especially in the rotavirus gastroenteritis-related cases, the frequency of neurologic abnormalities was higher, but had a favorable prognosis [
24].
Cho
et al. [
5,
23], and Lee
et al. [
6] described the clinical characteristics of CwG, as follows: 1) healthy children with ages mainly between 6 months and 3 years who had afebrile convulsions with gastroenteritis symptoms; 2) convulsions were often composed of clusters; 3) the blood electrolytes levels, blood glucose levels, and CSF test were all normal; 4) EEGs were normal; 5) neurological-developmental prognoses were very favorable. Choi
et al. [
3] and Choi and Suh [
25] suggested that the CwG should include convulsions associated with fever. In two comparative studies, the febrile convulsion group and the CwG group of Cho
et al. [
3] and Yoon
et al. [
4] showed differences in the frequency of recurrences within 24 h, in the family history of febrile convulsions, and in the rate of positive rotavirus antigen. Yang
et al. reported the genotypes of rotavirus and the Norwalk virus causing convulsions [
20].
In the literature, the mean ages of patients with febrile convulsions and CwG were between 12 months and 24 months [
7,
13]. In this study, the mean ages of patients in group B and C associated with AGE were 21.2 ± 22.0 and 22.0 ± 18.7 months, respectively. Our results were therefore similar to those found in previous studies. However, the mean age of group A was 28.3 ± 17.9 months, which was higher than the other groups, and also higher than the mean age reported in previous studies. There was a significant difference between group A and group B, but we found no significant difference between group A and group C.
Unlike febrile convulsions, CwG was reported [
17,
26] to be more prevalent in female than in male in some studies, but the prevalence was the opposite in another study [
5]. It is not clear why the ratio of male to female was controversial in the previous studies. In our study, the percentage of females was higher in group C, but there was no significant difference among the three groups. The incidence of family history of febrile convulsions was highest in group A, and there was significant difference in the incidence between group A and group C. The family history of febrile convulsions occurred in 31 (41.3%) of the 75 patients in group B and C. Among group B and C, 11 (30.6%) of the 36 patients with afebrile convulsions had a family history of febrile convulsions. These percentages were higher than the 4.3–27.3% that Uemura
et al. [
13], Yoon
et al. [
4], and Lee and Ong [
21] reported. In this study, the children who experienced more than twice the number of episodes of febrile convulsions had more prevalent family history of seizures [i.e. 70.1% (87 of 124 patients)]. This was higher than the average 52.5% (371 of 706) found in all of the investigated children. We assume that the relationship between family history and the incidence of recurrent episodes was significant. Nevertheless, it is possible that the taking of family history in the cases with first-time convulsions might not have been accurate. This study also included a relatively large number of children (217, i.e. 30.7%) with a past history of febrile convulsions. This might explain the finding that the mean ages of group A were higher than the ones of other groups. In addition, previous studies investigated only the family history of the parents, whereas this study included cousins and uncles. These reasons may have contributed to the high incidence of family history found in this study.
In the literature, the previously reported convulsion duration was between 5-and 10 minutes [
5,
17]. In this study, the frequency of convulsions with duration greater than 15 min was 6.3% in group C, 4.7% in group B, and 2.5% in group A, but there were no statistically significant differences among the three groups. The most prevalent clinical features of the patients with CwG were that the convulsions occurred in clusters or as multiples, and that they occurred within the first 24 h of hospitalization. In this study, there was a child who had eight episodes of convulsions within 24 h. The frequency of the recurrence of convulsions during hospitalization was higher in groups B and C than in group A, and this difference was statistically significant. After admission, recurrent convulsions occurred, on average, at 6.85 h and 2.40 h in group A and B, respectively, and the difference was statistically significant. Convulsions associated with gastroenteritis (CwG) (i.e. group B and C) showed higher frequency of afebrile convulsions than those in group A. Afebrile cases (i.e. children with convulsions associated with AGE) were more likely to show cluster types of convulsions than febrile cases. Uemura
et al. [
13] showed that most convulsions recurred within 24 h following admission. Therefore Yoon
et al. [
4] suggested that better management to prevent the recurrence of acute, repetitive convulsions within 24 h of hospitalization should be considered.
Although rotavirus gastroenteritis was more frequently associated with afebrile convulsions than non-rotavirus gastroenteritis, Lee
et al. reported that the age of occurrence, the tendency of recurrence, the convulsion characteristics, and the duration of convulsions were all similar between group B and group C [
27]. In this study, most clinical characteristics, except for the distribution of the month of admission and the sex ratio, were also similar. Therefore, the clinical characteristics of convulsions with AGE might be mostly similar, regardless of the type of causative virus. Therefore, it seems unimportant to strictly distinguish between them. In addition, the febrile cases of patients suffering from convulsions with gastroenteritis (CwG) are clinically similar to the febrile convulsions group (i.e. group A) with regard to the family history and past history of febrile convulsions and the frequency of cluster convulsions, compared to the afebrile cases of CwG. Thus we consider that the categories of CwG should be limited to cases of afebrile convulsion with gastroenteritis, similar to the classification used in previous studies.
Lloyd
et al. reported that in 11 (32.4%) of the 34 children suffering from convulsions produced by the rotavirus gastroenteritis, the convulsions were febrile [
28]. However, some observational studies [
29–
31] have shown that as much as 60–94.4% of children with rotavirus gastroenteritis had a fever. 717 (72.9%) cases of 983 patients with rotavirus gastroenteritis, aged 5 and under, who were admitted to the Sunlin hospital during the same investigative period, had a fever [
32]. The fever duration was relatively short, 1.8 days on average. The fever duration was shorter in many cases when the fever symptoms had presented before or after convulsions, but the body temperature at admission was 38.0°C and below. Therefore, the actual incidence of fever-associated cases might have been higher than the results of 60.4% for group B and 40.6% for group C, found in our study.
As the intensity of fever can be different, even in infections with the same viral strain, and the definition of fever is not standardized, various results are reported by different research groups, as previously seen in the literature. Cases that did not meet the fever definition of this study were found in 90 (14.3%) of the 631 children in the febrile convulsions group (group A). Similarly, in gastroenteritis produced by the same viral strain, children can present with diverse severity of diarrhea symptoms, according to the patients’ ages and their immunologic status. Furthermore, some patients present with vomiting without diarrhea symptoms. In other words, the diagnosis of fever and diarrhea might depend on the subjective judgement and the clinician’s bias, as stated previously.
Fever, which is a symptom that accompanies minor infections, like diarrhea, is thought to be a factor that helps to predict the clinical outcome, rather than an absolute criterion in differentiating the disease. In contrast, febrile convulsions and CwG are defined as convulsions provoked by minor infections, requiring healthy children of age 5 and under to be admitted for about three hospital days and showing a good prognosis. A more serious prognosis and the possibility of seizure recurrence during their hospitalization are fully explained to their guardians only in children suffering from convulsions with diarrhea, especially the afebrile cases. In these cases, active management of administering anticonvulsants within the first 24 h should be considered.
In conclusion, in our study, the prognosis of both febrile convulsions without acute gastroenteritis and of convulsions associated with acute gastroenteritis was favorable, with respect to the average number of days of hospitalization; however, there were differences in the distribution of the month of admission, the age of occurrence, the family history of febrile convulsions, the recurrence of convulsions during hospitalization, and the frequency of afebrile convulsions. In cases with convulsions associated with acute gastroenteritis, especially in afebrile cases, the cluster-type convulsions were more likely to occur within 24 h following admission.