Tuberous sclerosis complex (TSC) is an autosomal dominant disorder. The most of the mutation is caused by a mutation in either the TSC1 gene (encoding hamartin), with the locus on chromosome 9q34, or the TSC2 gene (encoding tuberin) located on chromosome 16p13.1,2 Epilepsy may be the most common prevalent and challenging clinical manifestation of TSC, and it occurs in more than 70–80% of the patients. Unfortunately, despite adequate antiepileptic drug treatment, many patients with TSC continue to have seizures.3 In most cases, tubers located in the brain are believed to be the epileptogenic focus, thus, epileptogenic cortical tubers are resected by epilepsy surgery.4,5 The overall seizure freedom rate after epilepsy surgery in drug-resistant epilepsy and tuberous sclerosis was 57% and seizure frequency improved by >90% in another 18% of patients.6 If a single primary epileptogenic tuber can be identified in patients with TSC, who were previously considered medically intractable, epilepsy surgery may be appropriate.6 Further on recent studies suggested that seizure freedom after surgery has been reported when both the tuber and the electrically active adjacent cortex were resected.7 We report two tuberous sclerosis cases of single epileptogenic focus, which can be the candidate for the epilepsy surgery.
Case Report
Case 1
The patient was a 30-year-old woman who had history of epilepsy since 3-year-old. She was transferred to our epilepsy clinic for the video-electroencephalography (VEEG) monitoring for presurgical evaluation. She was right handed, high school graduate, and unemployed. Physical examination revealed shagreen patch on her right lumbar region and few hypomelanotic macules on her back. Neurological examination revealed no remarkable neurological deficit. She reported to have monthly psychic and, or abdominal aura which often evolves to complex partial seizure, while on valproate, topiramate, carbamazepine, and pregabalin. Caregivers reported that she fumbles her right fingers and shows lip smacking during her seizures, and does not respond to questions. She reported only one episode of generalized tonic clonic seizure which occurred before taking anti-epileptic drugs. Caregivers also reported the change in the personality. She became aggressive and violent, mostly addressed to caregivers.
Intellectual assessment revealed IQ score of 83 which indicates low average level. Her neuropsychological test indicated bilateral frontal lobes and right temporal lobe dysfunction. Beck’s depression inventory score was 33 and Beck’s anxiety inventory score was 34, indicating severe depressive and anxious state.
An 8-day Video-EEG monitoring study demonstrated frequent interictal epileptiform activity localized to right frontotemporal region. Five seizures and one aura were recorded. Semiology was psychic, and, or abdominal aura which evolves to automotor seizure with right hand fumbling. Ictal EEG exhibited two seizures with maximum amplitude over the right frontotemporal electrodes, and three were obscured by artifacts. Remarkably, all five seizures had theta range rhythm build up at right frontotemporal electrodes (Fig. 1). The brain magnetic resonance imaging (MRI) study revealed multiple cortical tubers including a prominent on right anterior temporal cortex with loss of normal gray-white differentiation, and other scattered cortical tubers are located at right middle, and superior frontal gyri, right insula, left medial frontal and Lt parietal regions.
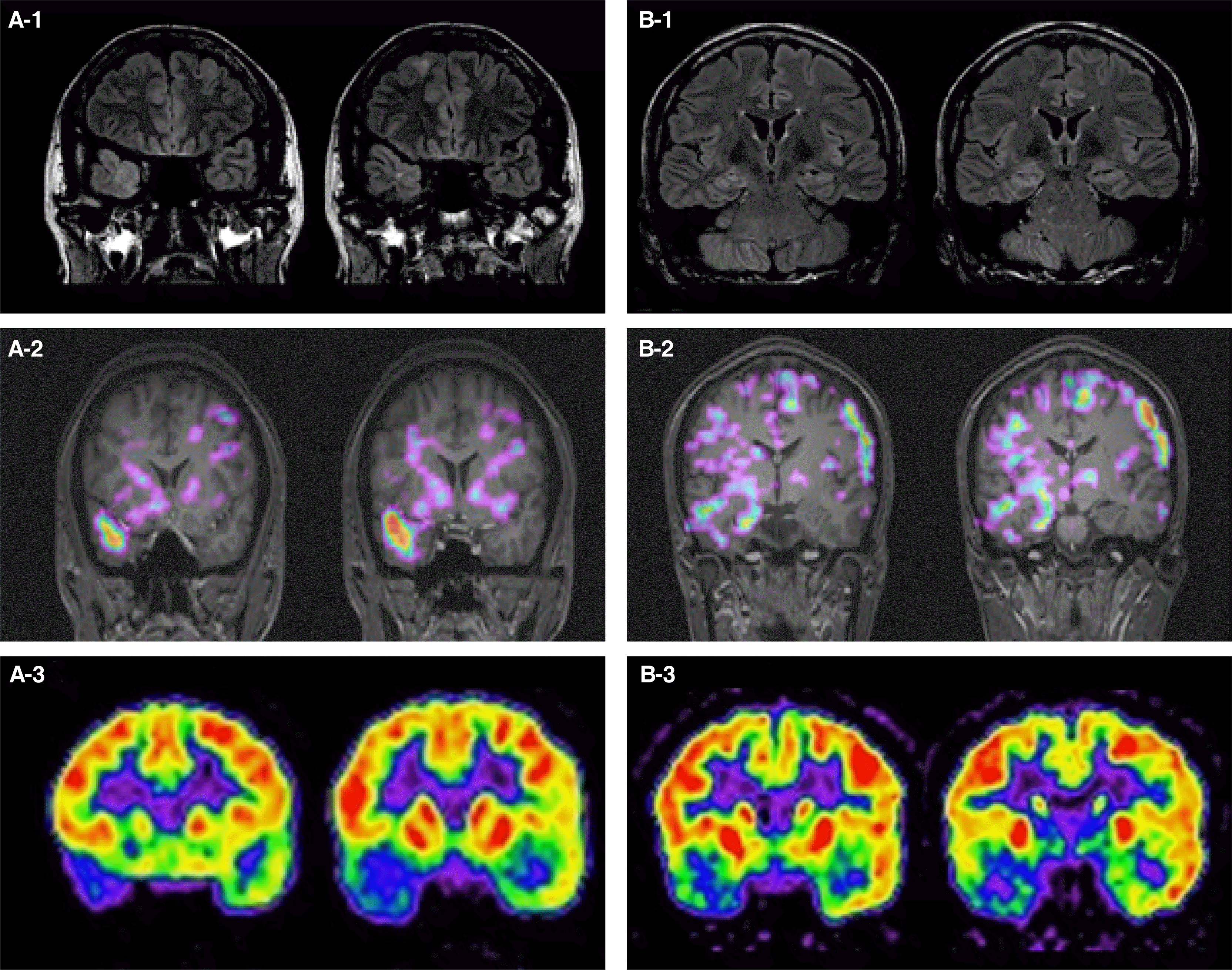
Radiotracer for ictal SPECT was injected at 24 seconds from the onset of 56 second duration habitual seizure. Ictal single photon emission computed tomography (SPECT) findings were concordant to the localization of ictal EEG onset. The hyperperfusion was regionalized to right anterior temporal area. This also anatomically correlated with cortical tuber seen at the right anterior temporal pole. Furthermore subtracted ictal-interictal SPECT co-registered with MRI (SISCOM) revealed hyperperfusion at the right temporal pole and anterior temporal region (Fig. 2A). Other cortical tubers seen in the brain MRI shows no definite regional perfusion changes. The ictal EEG at the timing of injection was rhythmic 5 Hz theta rhythm in right frontotemporal region. Brain 18F-fludeoxyglucose positron emission tomography (FDG-PET) reveals right anterior to posterior temporal regions hypometabolism, especially involving mesial temporal area (Fig. 2A). The genetic test confirms the gene mutation in TSC1 (c.2389C>T). Further evaluation for other symptoms of tuberous sclerosis including abdominal and thoracic CT scan were unremarkable.
Case 2
The patient was a 23-year-old male who had a history of epilepsy since 8-year-old. He was transferred to our clinic for the presurgical evaluation. He was bi-handed, college graduate, and unemployed. Physical examination revealed shagreen patch on his lumbar region, no other definite dermatologic abnormalities were seen. Neurological examination revealed no remarkable neurological deficit. He reported seizures consisting of automotor seizure and generalized tonic clonic seizure once in a year, and psychic auras several times per month while on valproate, topiramate, lamotrigine, and clonazepam. He reported infrequent complex partial seizures and secondary generalization. He also complained about the severe disability in concentration following polytherapy. He reported that seizures are provoked by loud music, thus he avoids earphone use or places with loud speakers, which withdraws him from normal social activities. Caregivers also reported the change in the personality and aggressive behavior since high school years. Intellectual assessment revealed IQ score of 108 which is average level.
During the six-day VEEG monitoring the patient experienced approximately five seizures and one aura. Semiology was psychic aura to unresponsive dialeptic seizure which then evolves to left versive seizure and generalized tonic clonic seizure. The video-EEG study revealed frequent interictal epileptiform activity, maximal over the right frontotemporal region with frequency of 3–6 per minute. These seizures all were lateralized to right hemisphere: three started from right frontotemporal area, and one from posterior temporal area (Fig. 3). Ictal discharges of all four seizures had theta range rhythm build up at right frontotemporal area. The brain MRI revealed multiple cortical tubers located at right hippocampus, inferior, and superior frontal gyri, left middle frontal gyrus, left mesial frontal cortex, left parietal cortex, and left occipital cortex. Radiotracer for ictal SPECT was injected at 34 seconds from the onset of 104 second duration habitual seizure. SISCOM revealed hyperperfusion at right temporal cortex (more on mesial) (Fig. 2B). Brain PET reveals right temporal lobe hypometabolism, especially involving mesial and basal cortex (Fig. 2B). He carried a disease-associated mutation in TSC1 (c.2578delG). His abdominal and thoracic CT scan showed no abnormal findings.
Discussion
This study reports two cases of partial epilepsy with localized epileptogenic focus in TSC with multiple tubers. Both patients’ first manifestation was epileptic seizure and was referred to our clinic at age of 30 and 23. Although both patients represented with right frontotemporal epilepsy, first patient had definite cortical tuber located at the anterior temporal pole, and second patient had cortical tuber at right hippocampus along with multiple cortical tubers.
The cortical tubers in tuberous sclerosis are potentially capable of generating epileptic seizures. Supporting this fact that, epileptiform discharges within cortical tubers were observed on intraoperative eletrocorticography,5 and surgical resection of tubers that have epileptiform activity has been associated with marked improvement in seizure profiles.5,8 Among multiple and widespread cortical tubers, identification for single epileptogenic tuber is critical for epilepsy surgery. There are no clear cut way to distinguish epileptogenic from non-epileptic tubers, by far the epileptogenic tuber is most often identified by concordance of interictal and ictal epileptiform activity with cortical tubers.8,9 Contrary to previous study, electrocorticography done in three tuberous sclerosis patients indicated that cortical tuber itself may be epileptically inactive, and that seizures may arise instead from the normal-appearing cortex surrounding the tuber by pressure effect or neurochemical changes.7 This might confuse where to be targeted for epilepsy surgery, but it also suggests that epileptogenic zone is adjacent to the cortical tuber. In case 1, our evaluation identified concordance of scalp EEG, SISCOM, PET, and MRI results for single epileptogenic tuber. Semiology of her seizure represented in two different types of auras, mostly psychic and, or abdominal aura. Abdominal auras are related to mesial temporal lobe epilepsy, and psychic auras are related to structures within the temporal lobes.10–14 Both of these auras have been reported in temporal lobe epilepsy, and the diversity of temporal aura indicates that temporal lobes contain various neural mechanisms relevant to psychic or cognitive functions. Surgical management may include grids, and strips covering adjacent areas, and depth electrode placed directly within the tuber to define where the epileptogenic area is.
In case 2, a high signal change of hippocampus in brain MRI indicates tuberous changes of gray matters of hippocampus. However, it is challenging to distinguish this signal changes from hippocampal sclerosis. Previous report demonstrated that prolonged seizures can lead to hippocamal sclerosis, which suggested rather extra-hippocampal lesion is the source of the very first seizures and these seizures are the initial precipitating injury that leads to hippocampal sclerosis, which sometimes referred to as “dual pathology”, or hippocampal sclerosis occurs as the consequence of an injury that causes both the hippocampal sclerosis and the associated brain injury.15 It is difficult in our stand point to identify rather hippocampal sclerosis is a “dual pathology” or subtle cortical changes in temporal region.
Despite multiple cortical tubers in TSC, epilepsy surgery is a therapeutic option for intractable patients, when the seizures originate from single focus. These seizure outcomes are lower than in patients with temporal lobe epilepsy surgery (66% seizure free), but higher than in patients with extratemporal lobe epilepsy surgery (27–46% seizure free).16 However, localizing the primary epileptogenic tuber, or focus are challenging. As in our cases, SPECT, with use of SISCOM can have important role in identifying the epileptogenic tuber.17