Introduction
Drug-resistant focal epilepsies are sometimes surgically remediable. After the first randomized controlled trial by Wiebe et al.1 in 2001 revealed dramatically improved seizure outcomes with epilepsy surgery over medical therapy in refractory temporal lobe epilepsy patients,1 the consensus guidelines started to recommend early surgical referral for pharmaco-resistant epilepsy patients.2,3
The purpose of hemispherectomy and hemispherotomy is to functionally isolate or eradicate the epileptogenic zone, which is widely diffused throughout the hemisphere. The first series of anatomical hemi-spherectomy for the treatment of gliomas was carried out by Dandy in 1928. The first anatomical hemispherectomy for the treatment of epilepsy was reported in 1938. In 1950, Krynauw4 reported that anatomical hemispherectomy, involving ligation of the anterior and middle cerebral arteries and subsequent en bloc removal of the cerebral hemisphere, was an effective surgical technique for infantile hemiplegia. However, this surgical technique has a high rate of late complications, such as obstructive hydrocephalus, chronic subdural fluid collection, and superficial cortical hemosiderosis, as was reported by Oppenheimer and Griffith5 in 1966. In order to avoid the severe complications of anatomical hemispherectomy, technical variations involving hemispherotomy with less resection and more disconnection have been described, such as hemidecortication by Ignelzi and Bucy,6 functional hemispherectomy by Rasmussen,7 and functional hemispherotomy by Delalande et al.8 The principle of functional hemispherotomy is leaving the live, vascularized brain, which is functionally disconnected from the contralateral healthy brain, intact. Hemispherotomy techniques were introduced in the 1990s by Delalande et al.,8 Villemure and Daniel,9 and Schramm et al.,10,11 each with their own solution to achieving the disconnections required to attain complete functional disconnection of the hemisphere. Cook et al.12 described a modified lateral hemispherotomy, which involves sacrifice of the middle cerebral artery with removal of a central block of opercular tissue. Bahuleyan et al.13 demonstrated the feasibility of a purely endoscopic transventricular hemispherectomy on cadaver brains as proof of concept. Hemispherotomy techniques involving partial cortical removal, which allow for the functional isolation of the hemisphere affected by severe epilepsy with excellent results, are continually being refined and have become predominant at most epilepsy centers in the 21st century. We retrospectively evaluated the outcomes, complications, and postoperative developmental status of patients who had undergone functional hemispherotomy for refractory childhood epilepsy.
Indications for functional hemispherotomy
Children with infantile hemiplegic epilepsy, which is characterized by unilateral hemispheric pathology resulting in refractory epilepsy, are potential candidates for hemispherotomy.14,15 Infantile hemiplegic epilepsy is not a single disease entity. A number of disorders, such as congenital neuronal migrational defects (e.g., cortical dysplasia, hemimegalencephaly, and hemiconvulsion-hemiplegia-epilepsy syndrome) and destructive lesions on the unilateral hemisphere (e.g., congenital porencephaly, perinatal cerebrovascular accidents, Sturge-Weber syndrome [SWS], and Rasmussen encephalitis) lead to intractable partial seizures and hemiparesis. Although wider resection or extensive disconnection is thought to result in optimum seizure control, these procedures increase the risk of neurological deficits as well as motor and mental problems. Thus, the surgical procedure for epilepsy must be selected by taking the pathology, semiology, developmental status, and age of the patient into consideration.
Surgical techniques for functional hemispherotomy
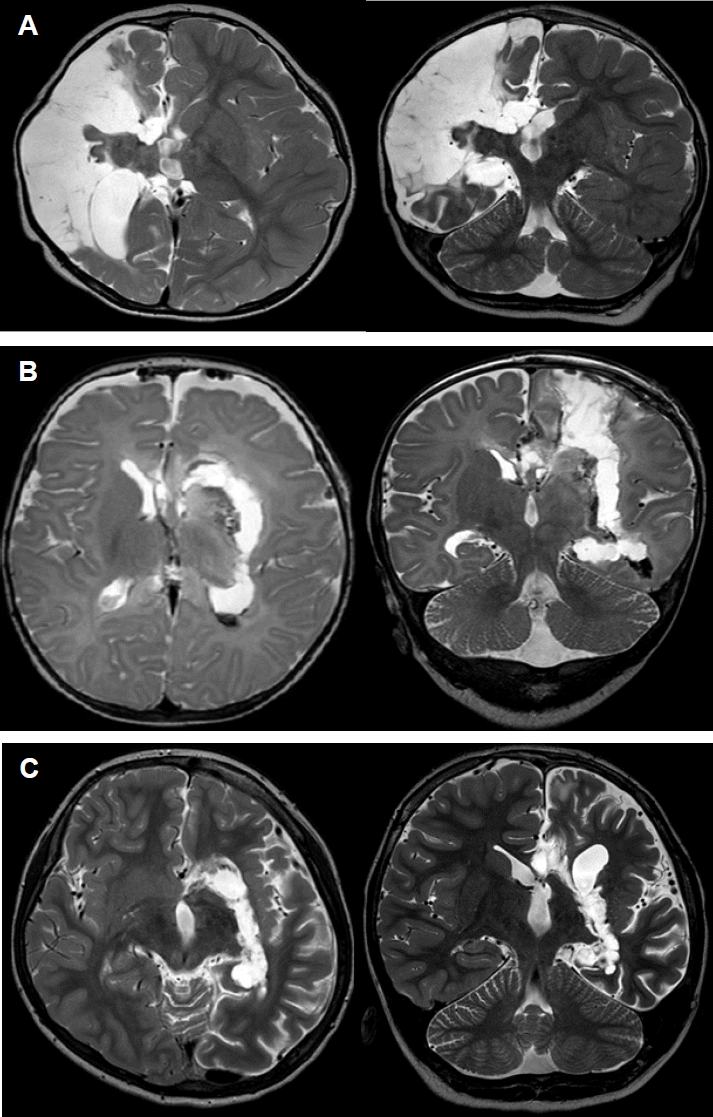
In order to completely isolate the pathologic hemisphere from the normal healthy hemisphere, anatomical hemispherectomy is the most precise method, except in cases with serious surgical complications. To achieve the same complete disconnection with minimal complications as in anatomical hemispherectomy, four common goals are necessary: disconnection of the cortico-thalamic tract (internal disconnection of the internal capsule and corona radiata), resection of the medial temporal structures, total corpus callosotomy, and disconnection of the orbito-fronto-hypothalamic tract (disruption of the frontal horizontal fibers).16 The two main surgical routes for functional hemispherotomy are a lateral approach via a surgical route around the Sylvian fissure (Fig. 1A) and a vertical approach that through the lateral ventricle and the corpus callosum from the brain vertex (Fig. 1B, C).
The “peri-insular hemispherotomy” technique9 is composed of three surgical stages: the supra-insular window, infra-insular window, and insula resection. The supra-insular window is able to reach the corpus callosum and dissect the white matter of the corona radia-ta from the frontal and parietal cortex (cortico-thalamic tract) via the lateral ventricle, preserving arteries and veins. After the callosotomy is completed along the pericallosal artery, dissection extends posteriorly to the hippocampus tail at the level of the choroidal fissure to the fimbria-fornix and anteriorly to the fronto-basal portion just anterior to the basal ganglia via the supra-insular window. The infra-insular window on the superior temporal gyrus allows for mesial temporal resection, including the uncus, amygdala, and hippocampus. The insular resection is completed by subpial aspiration or undermined by incising at the level of the claustrum.9
The general principle of the “vertical parasagittal hemi-spherotomy” is to achieve the same line of disconnection as achieved with peri-insular hemispherotomy through a posterior frontal cortical window (Fig. 1B) or the corpus callosum itself (Fig. 1C).8 We can reach the lateral ventricle with a small craniotomy similar to that used in a classic callosotomy. The dissection of the white matter of the corona radiata from the frontal and parietal cortex is done through the internal capsule lateral to the thalamus and continues from the trigone to the temporal horn of the lateral ventricle along the choroid plexus. Mesial temporal resection is then performed. The surgery is completed with the disconnection of the fronto-basal portion just anterior to the basal ganglia. This surgical technique provides a good anatomical orientation and allows for complete disconnection of the hemisphere while leaving the majority of the hemisphere, along with its afferent and efferent vascular supply, intact.
Preoperative evaluation
Presurgical evaluation includes: 1) family and personal history; 2) complete seizure history, including the onset of epilepsy, semiology and frequency of seizures, classified according to international league against epilepsy (ILAE);17,18 3) neurologic examination focused on motor and/or sensory-motor lateralized neurologic deficits (hemiparesis, unilateral hypotonic syndrome, and hemianopsia); 4) neuropsychologic examination; 5) 24-hour video electroencephalography (EEG); and 6) neuroimaging, such as brain magnetic resonance imaging (MRI), positron emission tomography and ictal and interictal single-photon emission computed tomography.
Results: seizure outcome, cognition, and complications
The seizure outcome for hemispherectomy and hemispherotomy is good in patients with acquired lesions and in patients with congenital malformations of cortical development, with a seizure-free rate of 60–90% and significant improvement occurring in about 10–15% of patients.10,13,19 However, seizure outcomes differ greatly according to etiology and surgical techniques.19,20 Rasmussen encephalitis, porencephaly secondary to perinatal stroke, and SWS have a better prognosis than cortical malformations such as hemimegalencephaly and cortical dysplasia, which may be associated with some degree of contralateral involvement.8 Some authors have pointed out that in terms of seizure outcome, anatomical hemispherectomy is the most effective because of the early recurrence of seizures due to incomplete disconnection in functional hemispherotomy. However, most neurosurgeons agree that the complication rate is higher with anatomic hemispherectomy than with the more recently developed functional hemispherectomy or peri-insular hemispherotomy. Seizure outcome has been reported to be poorer in patients with cortical malformations compared with patients with acquired lesions. Unintended incomplete disconnection is a well-recognized surgical outcome of lateral or vertical functional hemispherotomy in patients with severe cortical malformations.12,18 In patients with extensive cortical malformation, the technical constraints and difficulties involved in identifying anatomic hallmarks during surgery may be the reason for incomplete disconnection. Incomplete basal ganglia disconnection may also play a significant role in seizure recurrence after hemi-spherotomy in patients with cortical malformations.21–23 Another reason for poorer seizure outcomes in patients with cortical malformation is their younger age at surgery. Functional hemispherotomy, which requires brain retraction during surgery, is infeasible to perform in the underdeveloped brains of young children. Poor surgical vision may lead to incomplete disconnection. However, recently developed hemispherotomy concepts make the seizure outcome similar among the three procedures (anatomical hemispherectomy and lateral and vertical functional hemispherotomy).10,13,19 Even if seizure-free outcome does not depend on the surgical procedure, hemi-spherotomy techniques are highly recommended when insular and subcortical abnormalities are present.24
We can sometimes predict seizure outcome using preoperative EEG or MRI. Independent epileptic discharges from bilateral hemispheres indicate a less satisfactory outcome. In contrast, abnormalities of background activity over the good hemisphere or bilaterally synchronous discharges may be associated with a good outcome.25 In pre-operative MRI, contralateral abnormalities or abnormal hemispheres with extensive insular and subcortical heterotopic gray matter are also recognized as poor predictive factors for seizure outcome.26,27 Moreover, contralateral MRI abnormalities with malformations of cortical development are observed in 25–72% of children.28,29 Nevertheless, contralateral abnormalities may not contraindicate the use of hemispherotomy to decrease seizure frequency.29,30
Post-surgery cognitive function improves in most cases. The continuous epileptic discharges spreading from the malformed hemisphere to the “healthy” hemisphere suppress normal development of the brain, which causes mental retardation.31,32 Therefore, although seizures remain, their frequency reduction allows for an improvement in behavior, schoolwork, and employment capabilities. The amount of improvement depends on the etiology, postoperative seizure freedom, duration of epilepsy before surgery, and contralateral hemispheric dysfunction. A longer duration of epilepsy before surgery is associated with poor prognosis for the global outcome, especially for verbal communications abilities.8,19,31,33 Post-surgical seizure free outcomes were shown positive correlation with verbal language outcomes in children with a developmental etiology compared with acquired pathology group.33 A lack of post-operative cognitive improvement may be related to abnormal metabolism as well as MRI abnormalities of the “healthy” hemisphere.29,31,34
Most patients who undergo hemispherotomy suffer from a transient aggravation of hemiparesis. However, severe aggravation of hemiparesis or hemiplegia has never persisted long-term.8,19 Residual motor control is more severely impaired for hand functions than for walking.8,33
Coagulopathy, aseptic meningitis, infections, cerebral infarction, hydrocephalus, and superficial cerebral hemosiderosis are the early and delayed complications that develop after surgery. The most common complication is hydrocephalus requiring ventriculo-peritoneal or subduro-peritoneal shunting, which accounts for 10–50% of complications.8,19,31,35 Hemianopia, which is expected, is recorded in all surgical patients. About 10% of cases require second look surgery for seizure freedom because of persistent seizures and MRI evidence of incomplete disconnection.19,35 The perioperative mortality is higher than 1%.8,9,12,36 Even if early surgery allows for better neurocognitive and psychosocial development because of its advantages for developmental plasticity, i.e., the transfer of motor functions in one hemisphere or language capability in the right hemisphere, surgical consideration for younger children requires careful analysis of several age-related issues in comparison with adults. The small blood volume and severe cortical malformations that sometimes need larger resection in infants may be closely related to the higher mortality rate.36 Though rare, mortality may occur in patients with Rasmussen encephalitis before severe atrophy, in SWS, or in hemimegalencephaly or hypertrophic diffuse hemispheric dysplasia because of brain swelling and hemispheric infarct secondary to ischemia, or from interference with the arterial supply or venous drainage, which leads to intracranial hypertension and sudden death.8
Conclusions
Cerebral hemispheric disconnection surgery is a well-established treatment for intractable epilepsy secondary to diffuse, usually unilateral hemispheric disease that is intractable to medical therapy. Hemispherectomy or hemispherotomy may provide remarkable results in terms of seizure outcome and improvement in quality of life. It is important for the epilepsy surgeon to appreciate the individuality of each candidate for hemispheric disconnection and to apply the most suitable technique for that patient.