Introduction
Status epilepticus (SE) is a life-threatening neurological emergency that requires immediate medical intervention, and is associated with high mortality and morbidity.1,2 The annual incidence of SE in children is reported to be 10–40 per 100,000.3 Refractory status epilepticus (RSE) is a more severe variant of SE, and was previously defined based on duration (i.e., when SE persisted for more than 1 or 2 hours).4 Presently, the most widely-accepted definition considers RSE to occur when seizures persist despite the administration of two appropriate anticonvulsants at acceptable doses,4 and is estimated to occur in around 10–40% of patients with SE.4,5 RSE has been shown to be associated with higher mortality and more neurological sequelae.6
Pediatric data from western countries on refractory SE using the new definition are available.7 On the other hand, the studies on RSE from this region are few, and they are primarily in adults.8,9 In addition, no recent systematic reviews on pediatric RSE are available from India, although Indian studies of pediatric SE include some RSE patients.10 Thus, this study was conducted to study the clinico-etiological profile of children with refractory convulsive SE, and to compare their profiles and outcomes to those of children with SE.
Methods
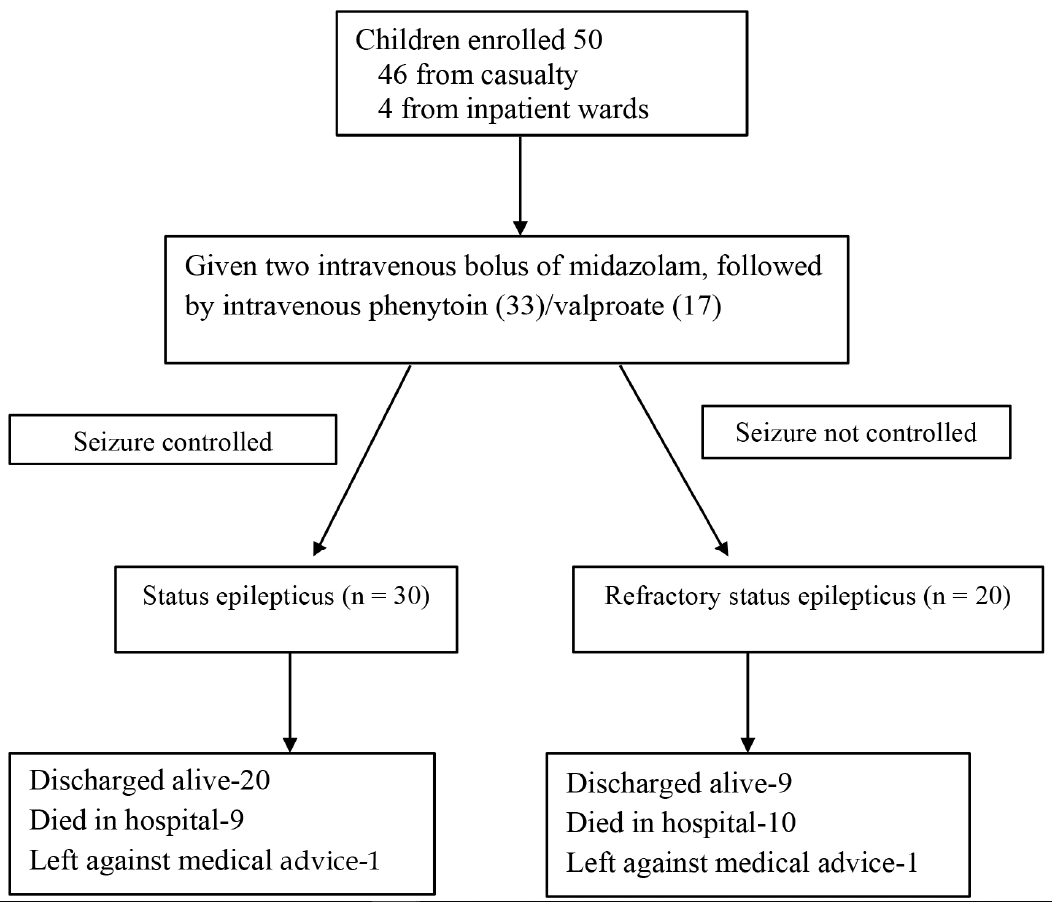
This longitudinal observational study was conducted in the pediatrics department of a large public hospital in New Delhi, India, over an 11-month period (1st April 2016 to 28th February 2017), after Institutional Ethics Committee clearance. Informed written consent was obtained from the parents of all participants. All children (aged 1 month to 12 years) who presented with a convulsive SE, or those who developed SE during the hospital stay, were enrolled prospectively (Fig. 1).
SE was defined as a seizure lasting longer than 30 minutes, or recurrent seizures that lasted more than 30 minutes, during which the patient did not regain consciousness.11 RSE was defined as seizures that persisted despite the administration of two appropriate anticonvulsants at acceptable doses.4,5 Outcomes were assessed based on the Glasgow Outcome Scale (GOS),12 and were applied when the patient left the hospital, whether discharged or left against medical advice (LAMA). A poor outcome was defined as death, persistent vegetative state, or severe disability (GOS, categories 1–3).
After initial management and stabilization, a detailed history was obtained from parents, which included seizure type, seizure duration, treatment given outside hospital, any precipitating factors, etc. In subjects with previously-diagnosed epilepsy, details of their previous diagnosis and management were noted from records, making a special effort to determine any history of non-compliance and/or changes in drug dosage. Any previous imaging results, electroencephalography (EEG) results, and antiepileptic drug (AED) levels were also documented. Relevant developmental history and significant past history were documented. A detailed systemic examination, as far as possible, was carried out in all children.
A provisional diagnosis based on the patient’s history and examination was made. A central nervous system (CNS) infection was defined as fever and seizures, along with features suggestive of meningitis, encephalitis or meningoencephalitis based on the hematological work-up, cerebrospinal fluid (CSF) analysis, blood/CSF culture, and neuroimaging. Febrile status was labelled when SE occurred in children (aged < 5 years) who had been previously diagnosed with febrile seizures.13
Children were managed according to the standard management guidelines.5,14 Briefly, this consisted of two doses of intravenous midazolam followed sequentially by intravenous phenytoin, valproate and levetiracetam. Additionally, intravenous phenobarbitone bolus, midazolam infusion, or ketamine were used in the intensive care unit (ICU). Airway, breathing, and circulation were maintained. Once seizures were controlled, neuroimaging and EEG were performed, and any additional required investigations were carried out. If the child was febrile, lumbar puncture was performed and CSF was collected for cytological examination, sugar-protein levels, and culturing. In patients in shock, for whom sampling studies were not possible initially, an initial diagnosis was made based on history and examination. Relevant samples were sent for analysis as soon as possible.
A detailed, structured form was filled out for each child, containing all of the above information. Children were monitored on a daily basis, with daily examinations and investigations performed as necessary. If beds were available in the pediatric ICU, children were shifted to the ICU. Otherwise, they were managed in the pediatric ward. Etiology and outcome were assessed in each child at the time of discharge/LAMA. The last examination’s details were used to determine the GOS score, in case of patients absconding from the hospital.
Statistical analyses
A convenience sampling size of 50 SE patients was decided a priori. All data were compiled in an excel spreadsheet and were analyzed using Epi Info software. Various clinico-etiological characteristics and outcomes were compared between children with SE and children with RSE. We carried out univariate analyses to determine the odds of RSE with respect to clinical characteristics at admission, abnormal neuroimaging, or abnormal EEG. Risk factors for a poor outcome were explored. A p value less than 0.05 was considered statistically significant.
Results
A total of 50 children (28 males) with SE were enrolled, of which 20 (40%) progressed to RSE. The median (interquartile range) age was 3.5 years (3 months to 12 years). Two children in each group showed seizure onset after being admitted to the hospital, while the rest presented to the emergency department during a seizure. The median (range) distance from the patient residence to hospital was 7 (1–15) and 5 (1–15) km for the children in the two groups.
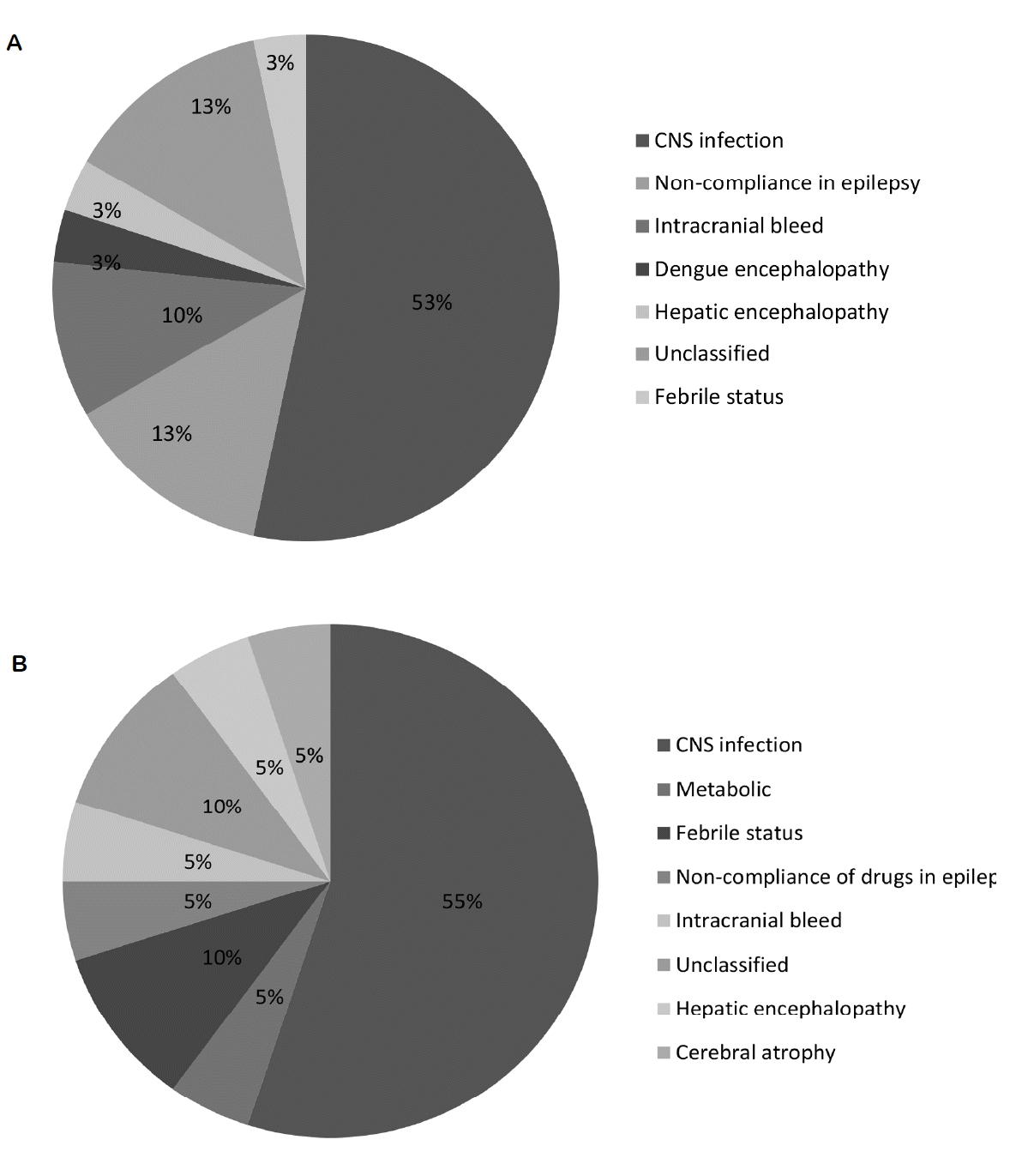
CNS infection was the most common etiology (53% in SE and 55% in RSE; Supplementary Table 1), and non-compliance with anti-epileptic drugs was the next commonest etiology, with no difference between the groups (Fig. 2). The proportion of patients with pre-existing epilepsy, the duration of epilepsy prior to SE, and the epilepsy etiology (idiopathic/symptomatic) were not significantly different between the two groups. The majority of patients (92%) had generalized seizures.
The risk of developing RSE was not significantly different between febrile children (odds ratio [OR], 1.17; 95% confidence interval [CI], 0.34–3.9; p = 0.8), children with epilepsy (OR, 0.69; 95% CI, 0.18–2.7; p = 0.6), children with developmental delays (OR, 1.25; 95% CI, 0.29–5.4; p = 0.8), partial seizures (OR, 5.12; 95% CI, 0.49–53.2; p = 0.17), microcephaly (OR, 1.62; 95% CI, 0.35–7.4; p = 0.5), shock at admission (OR, 0.67; 95% CI, 0.19–2.36; p = 0.5), or presence of a co-morbidity (OR, 1.76; 95% CI, 0.51–6.2; p = 0.4) (Table 1).
Nineteen (38%) children died during the study, with CNS infection being the most common cause of death (84.2%) (Table 2). The odds of death in children with RSE (50%) were higher than those with SE (30%), but the difference was not statistically significant (p = 0.15). A poor outcome (score 1, 2, or 3 in GOS) was seen in 56% of the subjects, with the odds being higher in children with RSE than in children with SE (OR, 6.0; 95% CI, 1.6–22.3; p = 0.005), as were the odds of being ventilated/intubated (OR, 9.67; 95% CI, 1.03–90.4; p = 0.047) (Table 2). Duration of seizure at presentation was not related to either the mortality (mean ± standard deviation [SD] duration: 35.3 ± 5.64 minutes vs. 34.8 ± 5.08 minutes; p = 0.78) or the outcome (mean ± SD duration: 35.9 ± 5.62 minutes vs. 34.1 ± 5.03 minutes; p = 0.2).
Discussion
In this hospital-based observational study of 50 consecutively-enrolled children with convulsive SE (including 20 with refractory convulsive SE) at a tertiary-care public hospital, we found that the majority of children showed new-onset SE. CNS infections were the most common etiology (54%) in both groups. A poor outcome was seen in 56% of the patients, and was significantly more common in those with RSE. The duration of seizure prior to presentation was not related to progression to RSE, or to a poor outcome.
The proportion of generalized seizures varies from 63–96% in previous pediatric SE/RSE studies,10,15–17 similar to our findings (85%). Around one-fourth of the study subjects in this study had a previous diagnosis of epilepsy, similar to recent studies on RSE from this region (16–29%).8,15–17 These data contrast with western pediatric RSE data, where rates of 47–50% are reported,7 possibly because of a higher proportion of SE in developing countries due to neurological infections.8,9
We were able to carry out EEG in only 66% of the patients, similar to previous experiences in both pediatric10 and adult8,9 Indians. Neuroimaging was only possible in 72% of patients, with 44% having a normal scan, similar to 36% reported by Sinha et al.8 The majority of studies from developing countries report CNS infections as the most common etiology of SE.8,9,16–18 Among pediatric studies,10 CNS infections are also the most common RSE etiology. On the other hand, in developed countries, the reported proportion of CNS infections as an etiology for RSE is low.6 Non-compliance with AEDs was an important precipitating cause of RSE in this study (13%), similar to previous reports on adults from this region (20–27%).9,19
A meta-analysis of pediatric RSE in 199920 reported a mortality rate of 16%; more recent studies report rates of up to 3.7%.7,15 Adult studies from neurological tertiary-care centers in India report mortality rates of 5–35% in RSE,8,9 whereas pediatric Indian RSE had a mortality rate of ~20% in two studies from the ICU of a tertiary-care center.16,17 We reported a higher mortality, which could be due to the smaller sample size, the decreased availability of intensive-care beds, or the more common acute CNS etiology.1 Among those discharged from the hospital, one-third were without sequelae in this study, similar to previous reports of pediatric RSE.6,15–17 However, around 50% of pediatric RSE patients in the meta-analysis by Gilbert et al.20 had a new neurological morbidity. Poorer outcome for RSE as compared to SE has also been reported previously.10,21,22 However, similar to a previous study from Korea,15 we did not find these differences. A higher frequency of death has recently been reported in RSE patients receiving the first dose of benzodiazepine 10 minutes after seizure onset.23 We could not assess this, as none of our patients had received the first medication within 30 minutes of onset, similar to reports of prolonged seizures prior to presentation in other Indian studies.19
In addition to the small sample size, one of the major lacunae of the study was the non-availability of neuroimaging and/or EEG data for some patients. This was either due to patient death, or to the family leaving the hospital against medical advice. We could only do short-duration EEG after SE control, and could only identify one NCSE patient. In contrast, De Lorenzo et al.24 reported 14% NCSE after SE, diagnosed on the basis of continuous EEG monitoring. The absence of ICU beds for all patients may have had an impact on the outcome, and prevents generalizing to a setting where SE is treated in the ICU. The major strength of this study was the consecutive enrollment of patients, and uniform, protocol-based management in all patients. This is the first study from a developing country that reports prospectively-controlled data on the evolution of pediatric RSE, using the newer definition of RSE.4
The high proportion of RSE patients with CNS infection as an etiology, the high mortality in the RSE group, and the poor outcome with respect to the presence of disability in the majority of survivors have important practice and policy implications. Early identification and management of SE and RSE in ICU settings could possibly reduce the mortality in this group. Given that the majority of SE and RSE patients had a CNS infection as etiology, the possibility of SE should be kept in mind and managed emergently in children with CNS infections. Other than neurological infections, the main etiology of SE/RSE in children with pre-existing epilepsy was non-compliance with AEDs; a situation that could be addressed by good patient counselling on treatment adherence. Further research may focus on prospectively identifying risk factors for SE to RSE progression in a larger patient group, and on following these patients for a longer period of time to define long-term outcomes with respect to disability, long-term seizure control, and neurodevelopmental outcomes.
In this hospital-based study of consecutively-enrolled children with convulsive SE (including 20 with RSE) at a public hospital, we found CNS infections to be the most common etiology and a poor outcome, especially in those with RSE. During the management of children with CNS infections, the treating physicians need to be aware of the high risk for developing RSE, and need to emergently manage this risk in an intensive-care setting, so as to reduce the mortality and morbidity from this severe neurological condition.