Introduction
Biotin-thiamine-responsive basal ganglia disease (BTBGD) is a rare, treatable autosomal recessive neurometabolic disorder caused by pathogenic variants in the SLC19A3 gene, which predominately affects children aged 3–10 years.1 BTBGD is more prevalent among Arab populations, mainly of Saudi origin.2 Three different phenotypes associated with SLC19A3 gene mutations are described: 1) early infantile leigh-like syndrome usually presents in the first 3 months of life with encephalopathy, and most of the affected patients have a poor prognosis despite treatment with biotin and thiamine; 2) classic BTBGD typically presents in early or late childhood with episodic of subacute encephalopathy manifesting as confusion, seizures, external ophthalmoplegia, and extrapyramidal symptoms; and 3) adult Wernicke-like encephalopathy presentation is characterized by the occurrence of confusion, ophthalmoplegia, ataxia, and seizures, which have a dramatic response to high doses of thiamine in the second decade of life.3 Pre-existing trigger factors were found in approximately 44.8% of the cases, including infections, trauma, vaccination, and strong physical exercise.4 The main differential diagnoses for BTBGD include mitochondrial disorders (leigh syndrome and pyruvate dehydrogenase complex deficiency), toxic encephalopathy, acute disseminated encephalomyelitis (ADEM), Wernicke encephalopathy, and inflammatory disease. Inherited disorders such as organic academia, Wilson disease, maple syrup urine disease, and Huntington’s disease should be considered in the differential diagnosis.3 BTBGD is not associated with specific biochemical criteria, which may help diagnose.5 The neuroradiological findings of BTBGD show classical features of bilateral symmetrical lesions in the putamen and caudate nucleus, and medial thalamus, with variable extension into the cerebral cortex, cerebellum, and brainstem.6 The initiation of treatment at the onset of symptoms is key to achieving significant improvement in patients with SLC19A3 pathogenic variants. BTBGD patients may require supportive care during an acute crisis, including intensive care. Patients with BTBGD should remain on biotin and thiamine for lifelong. During acute crises, the thiamine can be increased to double the regular dose and be given intravenously. Trihexyphenidyl, L-dopa, and clonazepam can be used to manage patients with dystonia. Rehabilitation therapy may be required to improve neurological outcomes and the quality of life.1,3,4 The prognosis of BTBGD depends mainly on the time of diagnosis, age at onset of symptoms, and underlying genetic variation.7
Here we report the clinical and radiological phenotype of a Saudi girl diagnosed with BTBGD based on a SLC19A3 gene mutation (NM_025243.4): c.1264A>G (p.Thr422Ala) by Sanger sequencing.
Case Report
Two-and-a-half-year-old Saudi girl with no significant past medical history presented to the emergency room with a 3-day history of frequent falls with an unsteady gait, tremors, slurred speech, and dysphagia with excessive drooling. On the 2 days of admission, the patient developed a lower-limb dystonic posture that severely limited her ability to walk and stand. There was no history of a decrease in the level of consciousness, seizure, or ophthalmoplegia. In the beginning, the mother denied any history of drug ingestion, trauma, recent infection, behavioral change, or neurodevelopmental regression, but after the toxicity test result, the mother mentioned that her daughter ingested around 20 mL of ethyl alcohol that is used in formulating perfumes 1 day before her presentation. According to the parents, the developmental milestone was uneventful and attained at the appropriate age. She was born to consanguineous parents, and she has an older brother who had a similar presentation, which was diagnosed as a case of BTBGD.
A physical examination showed her to be alert, conscious (Glasgow coma scale 15/15), and vitally stable, with no meningeal signs. She speaks in full sentences with dysarthria. Her pupils are equal, round, and reactive to light. Her extraocular muscles are intact, and her face is symmetric. A neurological assessment revealed normal power and deep tendon reflexes, along with a bilateral plantar flexor response. With daily assessment, the tone increased in the legs, which maintained an extended posture with ankle inversion and plantar flexion. She supports herself on her legs but was unable to walk unassisted due to ataxia. The tremulousness of both upper extremities was noted.
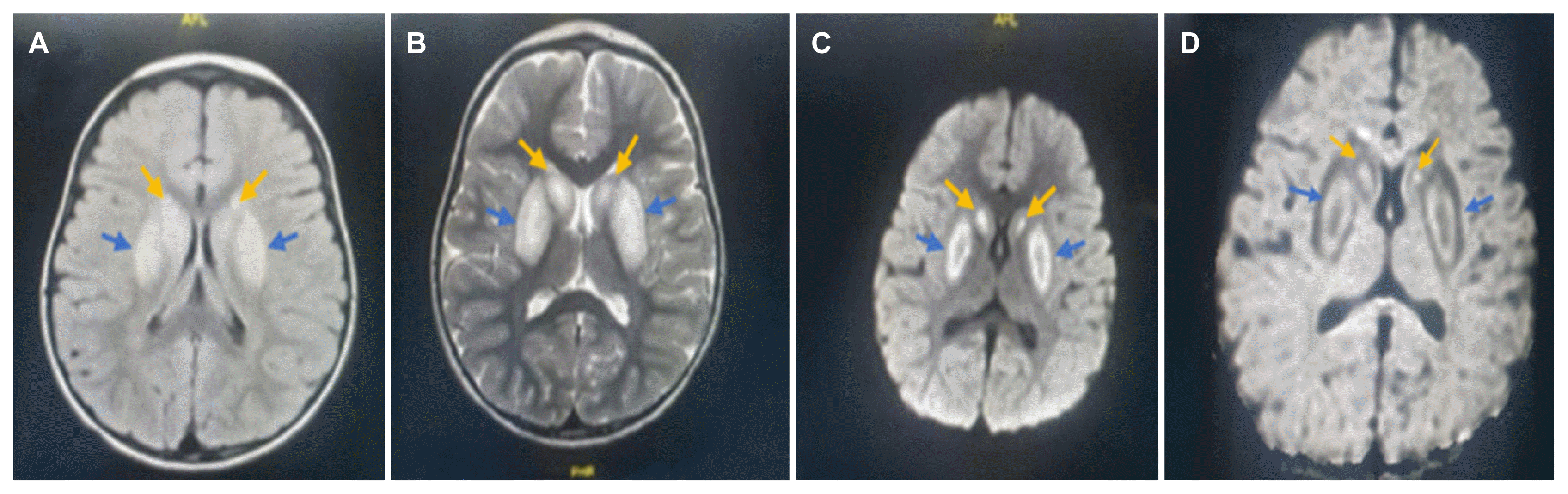
The initial impression was BTBGD due to the presence of a positive family history, but other possible causes such as toxic encephalopathy, ADEM, and mitochondrial disease were considered. The patient was admitted to a high-dependency unit for monitoring. The initial laboratory test results revealed a normal complete blood count, renal and liver function tests, and a basic metabolic workup. After a few days of admission, a toxicity test result came back positive for ethyl alcohol (10 mg/dL). Brain magnetic resonance imaging (MRI) showed bilateral symmetrical areas of abnormal signal intensity, which was high on the fluid-attenuated inversion recovery, T2 weighted image and restricted diffusion on the diffusion-weighted images and apparent diffusion coefficient noted in the basal ganglia in both putamen (blue arrows) and caudate (yellow arrows) nuclei. The cerebral cortex, subcortical white matter, cerebellum, and brainstem were relatively spared (Fig. 1).
Based on the clinical presentation, characteristic features on brain MRI, and positive family history of BTBGD, the patient started on 50 mg of oral biotin twice daily (8 mg/kg/day) and 200 mg of oral thiamine three times a day (50 mg/kg/day). Clonazepam 0.12 mg twice a day (0.02 mg/kg/day) was added for dystonia. Because the oral thiamine was temporarily out of stock, the patient received the 6th and 7th doses of thiamine intravenously, which showed marked improvement in slurred speech, dysphagia, and salivation. On a daily basis, there was slow and gradual improvement in her condition, but a significant and dramatic improvement was noted 1 day after thiamine increased to 300 mg three times a day (75 mg/kg/day). She started to walk alone for long distances with a normal gait and speak in full sentences without dysarthria, and there was no more dystonia. Only an unnoticed mild tremor was observed, which was bothersome and interfered with her daily activities at the beginning of the disease. Therefore, genetic testing was sent, and she was discharged on the 11th day of admission in good condition on biotin and thiamin and advised to continue biotin and thiamine for long life and to taper clonazepam over 1 month.
Discussion
BTBGD is an inherited neurometabolic disorder characterized by progressive encephalopathy. BTBGD is caused by pathogenic variants in the SLC19A3 gene and is more prevalent among Arab populations, mainly of Saudi Arabian descent. The mutation has variable penetrance, with clinical features ranging from normal to severely affected, and the disease course of affected individuals is highly variable as far as age of onset, treatment response, and outcomes. BTBGD has classic imaging findings characterized by bilateral symmetric signal hyperintensity and swelling of the basal ganglia during the acute phase and atrophy and necrosis in the chronic phase.1–3 Thiamine (vitamin B1) is a water-soluble vitamin that is an essential cofactor involved in multiple cellular metabolic processes.8 The human body is unable to synthesize thiamine, so a continuous dietary supply is necessary as it has a short half-life ranging from 1 to 12 hours and limited storage capacity (30 mg).8–10 In the absence of regular consumption, the thiamine storage is depleted within 2–3 weeks, and within 72 hours in the presence of acute illness.11 Thiamine monophosphate and free thiamine are absorbed in the small intestine by thiamine transporter-2 encoded by the SLC19A3 gene and thiamine transporter-1 encoded by the SLC19A2 gene.12,13 Mutations in any of these genes likely result in an impaired ability to transport thiamine into cells, leading to neurological dysfunction. In this report, we present an unusual case of a patient with BTBGD who developed the first symptoms after ingestion of ethyl alcohol and responded to a high dose of thiamine. As reported in the literature, the majority of BTBGD is triggered by infections (gastroenteritis and upper respiratory tract infections), trauma, vaccination, and strong physical exercise.4 However, it has not yet been reported that ethyl alcohol can trigger BTBGD in children, which may be due to the rarity of the disease, uncommon use of ethyl alcohol in children, or low ethyl alcohol concentration intake as it is widely used in household products and medicines as a solvent to improve drug solubility. We hypothesize that our patient is likely a carrier of BTBGD as she has a positive family history of BTBGD, which could be activated by ethyl alcohol ingestion. Our hypothesis is firmly supported by the fact that there was no other factor apart from the ingestion of ethyl alcohol. Moreover, there is a relationship between alcohol and thiamine deficiency, which can cause inhibition of intestinal thiamin transport, decreased conversion of thiamin to the active coenzyme, and impaired thiamin absorption.14 Furthermore, it is well known that alcohol consumption can result in thiamine deficiency, as in adult-onset Wernicke-like encephalopathy.15
Classically, administration of a high dose of thiamine may improve the symptoms within days. In the literature, it is recommended to start biotin (5–10 mg/kg/day) and thiamine (up to 40 mg/kg/day with a maximum of 1,500 mg daily) as early in the disease course as possible and continue lifelong.3 However, our patient only started to improve after administration of thiamine at (75 mg/kg/day). Patients with BTBGD should be cautious and aware of ethyl alcohol products, which can lead to a BTBGD crisis. The administration of a high dose of thiamin (75 mg/kg/day) may be required in patients who have not responded to the recommended dose. Further clinical research is required to determine the optimal doses.