Introduction
Deep brain stimulation (DBS) is commonly accepted as a safe and effective treatment for many neurological diseases including movement disorders, refractory epilepsies, psychiatric diseases and pain disorders.1–4 DBS of the anterior nucleus of thalamus (ANT) was recently reported as beneficial for patients with intractable epilepsy in a randomized, controlled, double-blind study.5
Many studies have discussed the complications of DBS on classical targets. However, because of limited experience with ANT DBS for epilepsy, its technical problems are rarely reported and unclear. In this case report, we describe a patient with a unique complication after ANT DBS, whose DBS lead had migrated into ventricle, detected 8 years after implantation.
Case
A 57-year-old male was admitted for progressive loss of anti-epileptic effect from bilateral ANT DBS during the last 6 months prior to admission. His first seizure developed at the age of 10. Since then, he suffered from chronic epilepsy with generalized tonic-clonic seizures and had regularly taken antiepileptic medications. A huge left frontal lobe meningioma detected due to increasing seizure frequency was removed in 2001, when he was 43 year of age. Seizures did not decrease. After about 6 years of multiple antiepileptic drug treatment by an experienced epileptologist in a tertiary hospital, he was diagnosed with intractable bilateral frontal lobe epilepsy. He was considered to have medically intractable epilepsy and was evaluated with presurgical studies, including video-electroencephalography (EEG), ictal and interictal single-photon emission computed tomography, magnetic resonance imaging (MRI), and positron emission tomography. Bilateral ANT DBS was performed in one hospital in 2007. According to the medical records and old brain images, the previous DBS had been performed with a bilateral transventricular trajectory. Bilateral DBS leads (Model 3387; Medtronic Inc., Minneapolis, MN, USA) and Soletra (Model 7426; Medtronic Inc., Minneapolis, MN, USA) pulse generators were implanted. He had undergone replacement of bilateral implantable pulse generators (IPGs) in 2011 due to IPG depletion. ANT DBS had been very effective for 8 years. The patient had one or fewer seizures a month. However, his seizure frequency had increased gradually to three or more times a month, since 6 months before admission. His left IPG battery was found to be depleted at the outpatient clinic 2 weeks before admission. Admission was decided to evaluate shortened longevity of the IPG and poor antiepileptic effect of DBS.
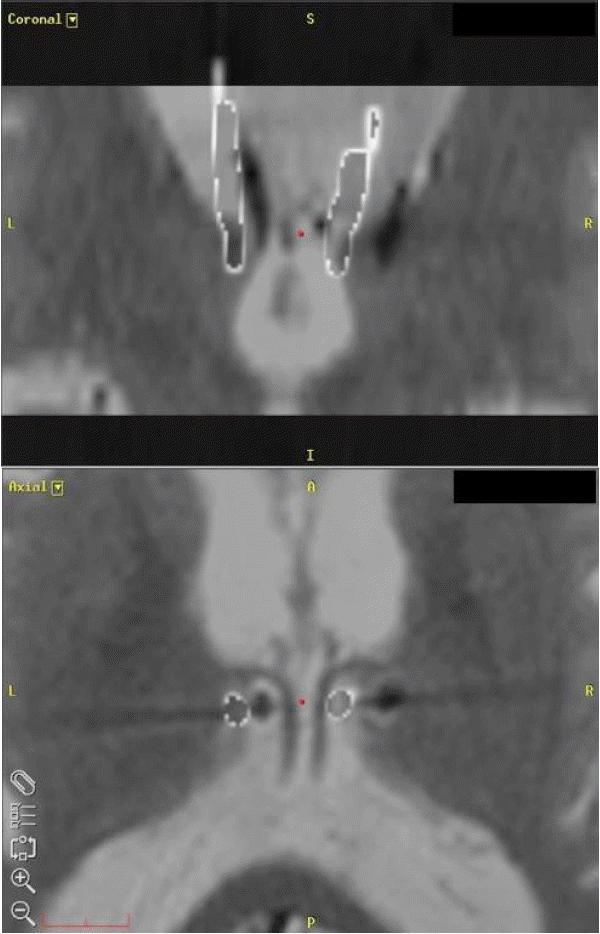
He had no major head trauma history that could have affected the function of the DBS system. Plain x-rays were taken to determine the integrity of bilateral leads and extension lines. Brain MRI showed that the left DBS lead was not located in the left ANT, but in the third ventricle (Fig. 1A). The third and lateral ventricles had enlarged significantly and bilateral Sylvian fissures had widened without any evident obstructive lesion in the cerebrospinal fluid pathway, compared to the MRI performed in 2007 (Fig. 2). We estimated the degree of ventricular enlargement and created fusion images using Framelink® navigation software (Medtronic Inc., Minneapolis, MN, USA) to identify the direction and degree of lead migration. To make fusion images, postoperative computed tomography (CT) scan, taken after the first DBS in 2007, was merged on the stereotactic MRI taken for re-visionary ANT DBS in 2015. Other areas in the CT scan, except the DBS lead, were made invisible by controlling the contrast and transparency of the image (Fig. 3).
The patient required re-implantation of a new DBS lead in the left ANT and replacement of the IPG. We removed the DBS lead and performed a MRI-guided, transventricular ANT DBS on the left side. Postoperative CT scan was taken for detecting any acute complication, and then it was fused with the preoperative stereotactic MRI to confirm the location of the electrodes. After verification of the EEG driving response and conducting test stimulations for 2 days, the IPG was replaced.
Comparison of the brain MRIs taken in 2007 and 2015 revealed that the Evans Index, the cella media index and the maximal width of the third ventricle had increased from 0.254 to 0.267, 0.142 to 0.204, 7.7 to 14.5 mm, respectively. The CT scan taken after the first DBS in 2007 showed that only the most distal electrode had definite contact with the ANT parenchyma (Fig. 1B), while the others were in the ventricle (Fig. 1C). We fused the CT scan with the recent MRI taken in 2015 using the navigation software. The fusion images showed that the left lead had migrated medially into the third ventricle and the right lead had migrated laterally during the intervening 8 years (Fig. 3).
After reimplanting the ANT DBS lead on the left side, the old DBS lead was explored. No sign of loosening or slippage was observed near the burr hole site. Rather, it was firmly attached to the burr hole with thick adhesions and new bone formation. After a simple adhesiolysis between the lead and surrounding tissue, the lead was easily removed without much risk of intracerebral hemorrhage as it was in the ventricle, rather than within the thalamic parenchyma. After removing the lead and the extension line, they were carefully inspected to confirm no hardware damage.
Postoperative CT scan taken after revision DBS showed no acute hemorrhage. The scan was merged with the preoperative stereotactic MRI to confirm the final electrode locations. The fusion image revealed that successful targeting was achieved with sufficient contact to the ANT parenchyma (Fig. 4). The patient showed a mechanical, antiepileptic effect after re-implantation of the left ANT lead, and a prominent EEG driving response was verified. After 2 days of test stimulation, the IPG was replaced. Chronic stimulation was provided with improved epileptic seizure frequency.
Discussion
The ANT is a relay station of limbic system in the human brain. It receives the mammillothalamic tract and projects to the cingulate gyrus.6 Electrical stimulation of the ANT to treat epilepsy was introduced by Sussman et al.7 and by Cooper et al.8 Recently, the SANTE multi-center, prospective, randomized, double-blind study (electrical stimulation of the anterior nucleus of the thalamus for treatment of refractory epilepsy) demonstrated the efficacy of ANT DBS to reduce epileptic seizures.5 The authors reported paresthesia, implant site pain and infection as the most common device-related adverse effects. Initially mislocated electrodes were 8.2% of their cases, but the details were not described.5
In the present case, it was obvious that the lead had spontaneously migrated due to the significant enlargement of the ventricle because the patient had no definite history of trauma and no securing failure was observed near the burr hole site intraoperatively. The CT scan taken immediately after the first DBS in 2007 showed that only the distal part of the left DBS lead had contact with the ANT parenchyma (Fig. 1B and 1C). The targeting was correct, but the contact surface for the electrodes was insufficient. In fusion images the left lead was revealed to have migrated medially into the third ventricle and the right lead had migrated laterally (Fig. 3). We speculate that the left lead probably moved laterally due to laterally acting pressure caused by enlargement of the ventricle. Then, the lead pulled out of the thalamic parenchyma, which eventually caused its medially directed migration, while the right lead remained in the laterally moved location in the thalamus. We think that it was caused by a shallow and insufficient lead implantation.
A transventricular trajectory is usually avoided in DBS for classical targets, such as the subthalamic nucleus, globus pallidus interna, and ventral nuclei of the thalamus.9 Ventricular puncture during the surgery may induce a brain shift due to loss of cerebrospinal fluid, which is a common cause of mistargeting. In addition, a DBS lead is not rigid enough to pierce through the ventricle and may proceed in the wrong direction in a curvelinear shape along the ventricular wall during the implantation.9 Moreover, many vascular structures in the ventricles can cause acute hemorrhage.10 Therefore, neurosurgeons carefully perform DBS so as not to interrupt the ventricular wall. However, transventricular trajectory is frequently chosen for ANT DBS because of its proximity to the ventricle.6 The medial dorsal nucleus of thalamus also has profound projections to the cerebral cortex, which can be affected by a more anteriorly located trajectory, and which may lead to a synergetic effect with ANT stimulation.11
During the 8 years, the patient had undergone significant atrophic brain changes (Figs. 2A and 2B). Because the human brain changes with time, it is important for physicians to understand and predict the absolute or relative changes in electrode locations, which may cause unexpected side effects or loss of benefits.12 According to a longitudinal cross-sectional study, the volume of the cerebral cortex and subcortical structures decrease, and the ventricular volume increases during healthy aging.13 In that study, the volumes of the inferior lateral (5.47%), lateral (4.40%), and third (3.07%) ventricles increased significantly, but the size of the fourth (0.71%) ventricle did not change.13 Studies have evaluated the influence of aging on structures, such as the subventricular and basal ganglia, the thalamus, and the brain stem region, where most DBS targets are located.13–15
The atrophic changes in the brain of a patient with epilepsy are more severe. In a volumetric MRI study, both the gray and white matter had more atrophy beyond the known epileptogenic area in the temporal lobe epilepsy (TLE) patient group, compared to that in a healthy group.16 Atrophy progresses more significantly compared to that observed during normal aging in patients with pharmacoresistant TLE.17 In the present case, in addition, surgery of the huge frontal meningioma in 2001, may have contributed the acceleration of atrophic changes of the brain by inducing traumatic injury. Therefore, regular brain imaging studies can be helpful to identify possible changes in electrode locations of ANT DBS, because many causes, such as aging, epileptic seizure and brain injury, can provoke and accelerate progressive ventricular dilatation and cerebral atrophy.
In conclusion, the progressive dilatation of the ventricles and shallow, insufficient implantation of the initial ANT DBS may have caused migration of the DBS lead. We recommend regular follow-up imaging studies as well as measuring impedance and battery status of the implanted device, because ventricular dilatation and cerebral atrophy can progress years after ANT DBS in an epilepsy patient with injured, atrophied brain.