Introduction
Ictal consciousness is a very useful concept in classifying seizures.1,2,3,4 Consciousness as a part of epilepsy was defined originally in work by Gloor.1 Consciousness in epilepsy is a very important subject and seizures pose a potential health care burden solely on the basis of preservation or lack of consciousness. There have been several studies so far addressing the role of consciousness and looking into the neurobiological and neuro anatomical substrates for the same.5,6 Various tools have been developed for the quantitative assessment of consciousness in seizures like the ictal consciousness inventory (ICI), responsiveness in epilepsy scales (RES), and the consciousness seizure scale (CSS).7 The Ictal consciousness inventory is a self-administered tool with 20 questions addressing two domains of consciousness.8 The questions 1–10 of the inventory assess the level of consciousness or awareness during seizures. This level of awareness is generally what is visible during seizures and falls along a continuum from minimally conscious to being in a fully alert state. The second part of the inventory is the content of consciousness which is the description of the subjective experiential phenomenon.5
Focal epilepsies by virtue of relative preservation of consciousness tend to have lesser involvement of the extensive thalamo-cortical circuits. This would also help classify seizures based on their propagation types. The temporal and extra temporal epilepsies have different propagation patterns and thus have varying level consciousness during seizures.2,8 The alterations in the contents of consciousness during seizures are thought to be determined by the specific motor, sensory, memory and emotional systems. This is determined by the activation of the thalamo-cortical and the brainstem systems.9 The ictal propagation of seizure activity as determined by intracranial electroencephalography (EEG) recording is different in mesial temporal, neocortical temporal and extra temporal epilepsy.10 Relative consciousness preservation in focal epilepsies is probably explained by this. We could then hypothesize that among the temporal epilepsies there should be difference in the level and content of consciousness between the mesial and the neocortical temporal epilepsy subtypes. We intended to study this population group and compare them with the extra temporal epilepsies.
Methods
This was an observational study design with three groups of persons with drug refractory epilepsy (DRE). The three groups were extra temporal, mesial temporal and neocortical temporal. The study was approved by local ethics committee. Patients were prospectively recruited from 2012 to 2014. The determination of epileptogenic zones and the classification of subjects in the three groups were based on semiology, imaging, and long term surface video EEG monitoring. The ICI was used.8 The phenomenology of the Ictal consciousness inventory was translated into Hindi and then back translated to English by the author and colleagues and was checked for content by language experts. The inventory was checked for external and internal validity. This inventory was administered to all patients admitted to the epilepsy monitoring unit (EMU) by the technicians. The ICI a 20 item questionnaire was used to calculate the scores for level (ICI-L, question 1–10) and content (ICI-C, question 11–20) of consciousness.
Definitions used in the study
Epilepsy was defined as per standard criteria in patients who had two or more unprovoked seizures (Commission on Classification and terminology International League against Epilepsy, International League Against Epilepsy [ILAE], 1981, 1985).11 Focal epilepsy was defined as per the latest ILAE (2010) terminology as those without impairment of consciousness or awareness, those with observable motor or autonomic components and those only involving subjective sensory or psychic phenomenon (Berg 2010).12 Drug refractory epilepsy was as defined by task force of ILAE.13
Results
There were 45 persons with mesial temporal (MTLE), 47 with extra temporal (ETLE), and 11 with neocortical temporal epilepsy (NTLE). Persons with epilepsy admitted to the EMU were classified into these three groups based on semiology, imaging and video EEG onsets. The demographic characteristics of these subjects are summarized in Table 1. All the patients were on varying combinations of levitiracetam, phenytoin sodium, clobazam, carbamazepine or oxcarbazapine. None of the patients were on Phenobarbital. Only 10 patients out of the total 103 (9%) received sodium Valproate. 39 out of 47 (82%) ETLE, 30 out of 45 (66.66%) of MTLE and 9 out of 11 (81.8%) of NTLE had secondarily generalised seizures whereas. The characteristics of the surface EEG in persons with mesial temporal type of epilepsy is summarised in Table 2. The extra temporal group had a higher number of persons with frontal than occipital epilepsy (78% vs. 21%) (Table 3). The scores ICI-L and ICI-C were compared between ETLE with MTLE and MTLE with NTLE.
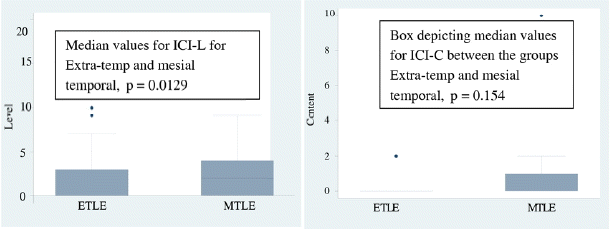
The level of consciousness among persons with mesial and extra temporal epilepsy was compared. Persons with mesial temporal had higher scores on the ICI-L questions (p = 0.0129). The content of consciousness did not differ in the two groups (p = 0.154) (Fig. 1).
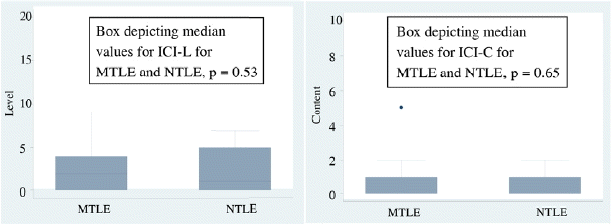
The level of consciousness among persons with mesial temporal and neocortical temporal was then compared. There was no difference among the two groups (p = 0.53). The content of consciousness also did not differ between the two groups (p = 0.65). (Fig. 2)
Discussion
This is probably the first study comparing the two groups of temporal epilepsy (mesial and neocortical) separately with extra temporal epilepsy. The three groups were compared among themselves for the total score each on the two sets of the questionnaire. Persons with mesial temporal had higher scores than the extra temporal group on the level of consciousness of the inventory (ICI-L). However the content of consciousness did not differ. This could be explained on the basis of the involvement of focal cortical regions in these patients. Blumenfeld et al. 2 in his paper on consciousness in epilepsy classified these as focal aware conscious seizures with relative preservation of level of consciousness. Level of consciousness in epilepsy is a continuum from minimally awake to a fully alert state. This in turn is determined mainly by the involvement of the brainstem and the thalamus.5 Patients with mesial temporal epilepsy by virtue of their spread which is different from extra temporal epilepsy differ in this and hence probably have a relatively preserved level of consciousness.14,15,10 Extra temporal epileptic seizures by virtue of their rapid spread and involvement of wider networks would hence lead to lesser level and content of consciousness scores. These seizures hence would rightly classify as focal seizures with impaired awareness.6 Also in the ETLE group there were higher numbers of patients with frontal epilepsy (78%). This also contributed to the differing level of consciousness in the two groups.
As highlighted by Cavanna et al.5,16 the content of consciousness comprises of mainly subjective experiences and their description. It is further described that the content of consciousness is mainly dependant on the environmental factors and the individual’s narration or recall of it and hence is highly variable. The relationship between the level and the content of consciousness is complex and yet to be better described. However with a heightened level of consciousness there should be better account and description of these experiential phenomenon. Indian patients by virtue of the cultural and lower educational level with limited narrative capacity are probably not much forthcoming about the content of consciousness. The lack of difference in the content of consciousness in the two groups despite a heightened level of awareness in the MTLE group could probably be explained by this. This finding could also be due to the small number patients we had in the study and the true picture would evolve only after larger studies with bigger sample of population.
We further classified the temporal group into mesial and neocortical temporal to see if there was any difference. There was no difference observed between the mesial and neocortical temporal groups. Blumenfeld et al.17 observed that arousal and alertness in temporal group of epilepsies is impaired not only because of the involvement of limbic structures but also due to bilateral cortical involvement in the form of fronto-parietal slowing, and contra lateral spread as has been observed in the EEG studies in this patient population. He also stressed on the role of slow cortical activity in these patients. Neocortical temporal and mesial temporal epilepsies however differ little in their mode of generation of experiential phenomenon.18 This is likely based on the reciprocal connections in the two.19,20 This we cite as the probable explanation for the lack of difference in the two groups in both the level and content of consciousness. Also there were smaller numbers of patients in the neocortical temporal group. This was probably due to lesser lesional NTLE patients getting connected to the video EEG, unless there was a lesion in the eloquent cortex. Also being a tertiary health centre, many MTLE patients get referred for surgical management outnumbering the rest.
Our study had some limitations; we could not compare equal number of patients in each group. However despite this and in the light of all the recent work on ictal impairment of consciousness we feel a study with a larger sample size would answer. the question.2,21 The recent role of resting networks and their role in specific areas of cognition as shown by functional studies may also be unique in each type of epilepsy.22 Also classification of seizures based on the ictal impairment of consciousness would seem justified based on these findings. The ICI was confirmed to be an informative and easy to administer tool for evaluating both the level and content of ictal consciousness.