Introduction
Hemiplegic Migraine (HM) is a rare type of migraine which involves reversible motor weakness and other aura symptoms. The disease is divided into familial and sporadic form by genetic studies or clinical history. Although gene mutation such as CACNA1A, ATP1A2 and SCNA1A were known to be related with familial hemiplegic migraine (FHM), there are no special diagnostic tool or guideline for sporadic hemiplegic migraine (SHM).1
A recent study has suggested that Electroencephalogram (EEG) may be used for determining charateristics of migraine and reported that increased beta band of T3, F7, O1 and O2 channels in migraine patients.2 Other literature presented that patients with migraine showed more common spikes than the control group. During attacks of basilar migraine, unilateral or bilateral delta waves are recorded frequently.3 As in cases with HM, there have been a few findings of EEG reported as slow sharp waves and diffuse slowing with epileptiform discharges during hemiplegic migraine attack.4–6 However, these EEG of hemiplegic migraine were performed without sleep deprivation (SD). In the present case, we report a SHM patient who showed specific EEG findings after SD that were not observed during routine EEG without SD.
Case
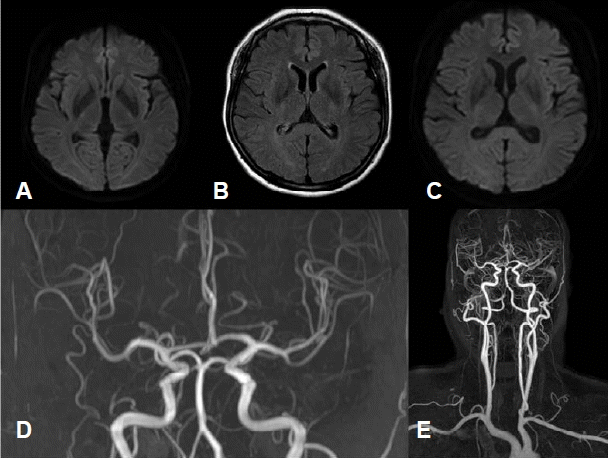
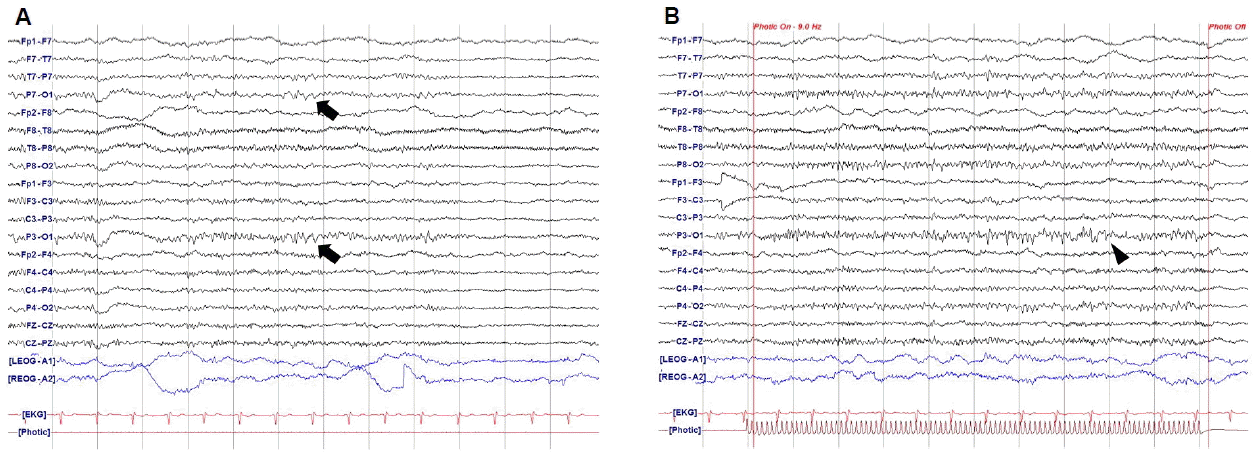
A 52-year-old women visited our hospital due to severe headache during the evening mass. She had a history of migraine for two years that occurred about ten times per year. The headache was accompanied by symptoms of right arm weakness, confusion and motor aphasia which started with a duration of one year. These symptoms started with the headache simultaneously and usually persisted less than a few hours. On admission day, she complained with pressure-like pain on the head diffusely that was aggravated by loud noise or activity of daily living. Her headache was accompanied by right arm weakness and mild motor aphasia that were developed simultaneously. She had no family history of HM, and the CACNA1A gene mutation was negative. Neurologic examination showed right arm paresis (MRC grade IV~V-), mild confusion and motor aphasia. The magnetic resonance imaging (MRI) revealed no specific findings except focal vascular dilatation on the right posterior communicating artery (Fig. 1). Her headache and aura subsided gradually and disappeared after 4 hours. She was admitted for further evaluation of recurrent headache. She had been taken medical treatment for prophylactic and symptomatic control with propranolol 10 mg twice a day, flunarizine 5 mg once a day, tofisopam 50 mg twice a day, tramadol 37.5 mg and acetaminophen 325 mg daily. She underwent total five times of EEG including two times that had been done before admission and only the last EEG was performed with SD. Every EEG was performed in symptom-free state for about 30 minutes, including activation process such as hyperventilation and photic stimulation. The previous four EEG revealed no specific abnormal findings. However, the last EEG which was performed after SD showed intermittent medium amplitude of 5–6 Hz theta slowing on the left parieto-occipital area which was also partially seen during photic stimulation (Fig. 2). Additionally, more prominent large amplitude of POSTs was observed on the left occipital area during sleep stage I (Fig. 3). Her headache was resolved during hospitalization and she became symptom free after three months of follow-up.
Discussion
The patient in the present case suffered from headaches accompanied by reversible motor aphasia and right arm weakness, lasting a few hours that satisfies the criteria of HM. We could diagnose the patient as SHM because of the aforementioned symptoms and the absence of familial history with HM (Table 1). Gene mutations of CACNA1A, SCN1A, and ATP1A2 can cause FHM and we investigated only CACNA1A gene mutation in the present case that revealed negative result.7 However, gene mutation is not one of the imperative criteria for SHM according to the beta version of the third edition of International Classification of Headache Disorders.1 The pathophysiologic mechanism of HM is not well known. Cortical spreading depression is an important factor to explain HM. Intracellular calcium influx significantly increased in the cerebral cortex of a CACNA1A-transgenic mouse. Higher concentration of intra-cellular calcium induces more excitable neuron which may result in excitotoxicity.8
Specific EEG findings about HM patients are known to be characterized by slow sharp waves on the hemisphere contralateral to the hemiplegic limb.4 Other researches showed diffusely slow and polymorphic theta activity with some epileptiform discharges over the cerebral hemisphere contralateral to the symptomatic hemiparesis.5,6 Recent report revealed the temporospatial dynamics of EEG during the full duration of a sporadic hemiplegic migraine attack. They suggested that EEG slow waves may reflect recovery of cortical spreading depression and large amplitude of slow waves during hemibody pain may be due to vasodilation of arteries.9 The EEG findings of the present case such as slow waves on the contralateral side of symptomatic limb and high amplitude of POSTs may also be explained by cortical spreading depression or vasodilation of arteries. However in the present case, the specific EEG findings revealed only after SD. Todd et al.7 reported SHM with permanent neurological deficits after sleep deprivation. They could not found the cause of permanent neurologic deficits except suggesting irreversible neuronal damage and did not focus on sleep deprivation of the patient. SD may be a burden in brain functioning and might have induced severe hemiplegic migraine or EEG changes in both cases. We tried to search other possible factors that might affect the EEG changes after SD. However, she had neither structural lesions on brain MRI nor histories of taking antiepileptic or antipsychotic drugs.
Detection rate of interictal epileptiform discharges (IEDs) in patient with epilepsy are increased after SD that is explained by stabilization of sleep instability and EEG cyclic alternating pattern.10–12 This case showed specific EEG changes in symptom-free state of SHM after SD. As the IEDs in patient with epilepsy are increased after SD, this may be also applied to SHM which could help clinical diagnosis.