Lennox-Gastaut syndrome (LGS) is a difficult-to treat type of epilepsy characterized by multiple types of seizures, generalized slow spike and wave activities or generalized paroxysmal fast activities in EEG, and progressive cognitive deterioration.1 Because medical treatment usually fails to achieve satisfactory seizure control, multimodal management is often required. Surgery for this condition includes callosotomy, vagus nerve stimulation or resective surgeries for limited cases with focal lesions.2,3 Among them, corpus callosotomy is effective especially for treating drop attacks which are the most notorious type of seizures in LGS.4,5 In some medical centers, anterior callostomy is often performed in order to avoid the complications of a disconnection syndrome, however, approximately 30% of these patients experience relapse of their seizures.6 In those cases, a second total callosotomy can be a useful treatment option. We report here the case of a patient with LGS who underwent staged total callosotomy, after which he achieved a seizure- free state.
Case Report
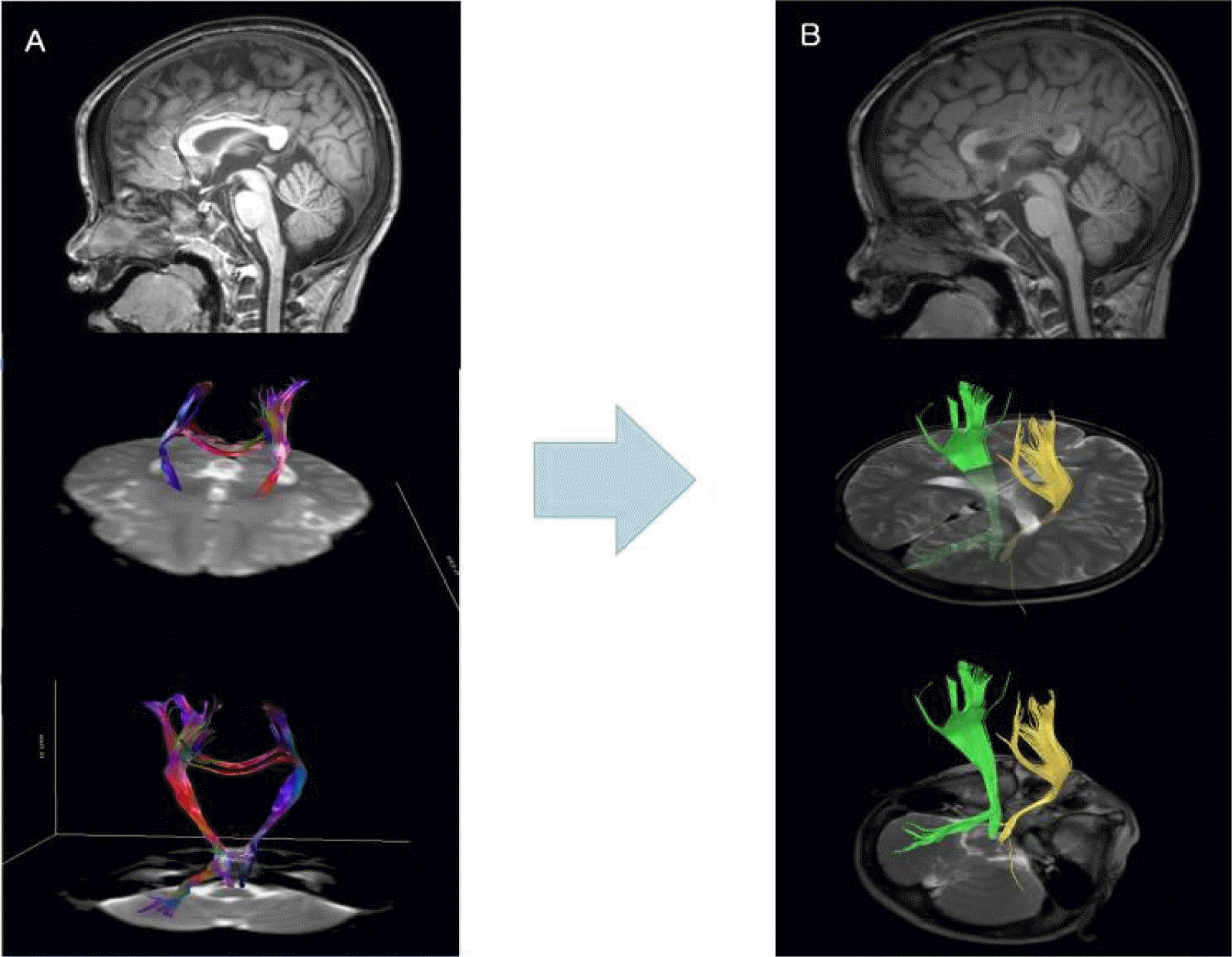
A 6 year-old boy was born at 34 weeks’ gestation to healthy parents with birth weight of 3,540 g after an uneventful pregnancy. He had no family history of epilepsy or focal neurologic sign, but he had delayed development in fine motor and language areas since before 12 months of age. His first seizures, myoclonic seizures, occurred at the age of 8 months, and he was started on antiepileptic drugs (AEDs). At the time of his admission to our hospital at the age of 27 months, he was suffering from multiple types of seizures including drop attacks, generalized tonic seizures, and atypical absence seizures. His EEG showed generalized slow spike and wave discharges with multifocal spikes (Figure 1), and he was diagnosed as having LGS. Brain magnetic resonance imaging (MRI) showed no abnormalities. Despite various antiepileptic drugs (valproate, topiramate, zonisamide, and clobazam) and a ketogenic diet, his seizures remained uncontrolled. Long term video EEG monitoring for 48 hours showed a dozen seizures a day. The seizure semiology consisted of suddenly dropping his head with losing his tone, thus suggesting myoclonic-atonic seizures. Ictal EEG showed diffuse generalized bursts of polyspike discharges with very brief electromyographic activities. His social quotient was reported to be 27. At the age of 36 months, he underwent anterior 2/3 corpus callosotomy. He achieved a post-operative seizure-free state for 3 months, after which he developed a relapse of his seizures. EEG performed at post-operative 10 months revealed frequent generalized spike and wave discharges and generalized paroxysmal fast activities. His seizures did not respond to either additional vagus nerve stimulation or to medical treatment at that time. As diffusion tensor imaging showed remaining callosal fibers in the splenium, we decided to perform a second total callosotomy (Figure 2). After the second total callosotomy, the patient maintained a seizure-free state for 27 months and with EEG normalization (Figure 3). He is still on the medication of valproic acid, topiramate, zonisamide, and clobazam. Developmental test performed after the second surgery showed no remarkable progress by that time. However, according to his parents, his cognitive function is improving compared to that of his pre-operative state.
Discussion
Corpus callosotomy is a palliative surgical procedure for patients with intractable seizures who are not amenable to focal resection. Since Van Wagen and Herren first performed commisurotomy in human in 1940, it is currently generally accepted that callosotomy is effective for disabling generalized seizures, particularly drop attacks.4,6–8 In drop attacks, 80–92% of patients who underwent corpus callosotomy achieved >50% seizure reduction, and 50–61% had >50% seizure reduction in generalized tonic clonic seizures.4 The rationale underlying diminishing seizures by corpus callosotomy is based on the hypothesis that corpus callosum is the largest commisure interconnecting the two hemispheres and that it has an important role in spreading interhemispheric epileptic activity.5 The anterior callosum carries fibers from the premotor, motor, anterior insula, and anterior cingulated gyrus, that is essential for the generalization of tonic and tonic-clonic seizures, and atonic seizures.4,5 On the other hand, splneium, the posterior callosum, connects fibers from the superior parietal lobe and occipital cortex that transfers perceptual information. Therefore, total callosotomy including splenium has a greater risk of complications, such as disconnection syndrome, due to the disruption of the transfer of visual, tactile, and auditory information.9
According to the previous studies, the optimal range of callosal section has not been clearly determined. Spencer et al.10 reported that anerior callosotomy was effective for secondarily generalized seizures in 35%, whereas total callosotomy was effective in 77%. Sunaga et al.6 also demonstrated that control of drop attacks was achieved in 76% of their patients with total callosotomy and in 54% of those with anterior callosotomy. They also reported that patients with anterior callosotomy had a higher relapse rate than patients with total callosotomy (31% vs. 7%). However, Mamelak et al.11 reported that anterior 1/2–2/3 callosotomy is nearly as effective as extensive total callosotomy for reducing both drop attacks and generalized tonic clonic seizures. They proposed that the extent of callosotomy is not an important factor if at least 50–65% of the callosum is divided.
Considering the seizure outcome and the risk of complications, some centers prefer to perform an anterior callosotomy which first spares the splenium, and then to perform total callosotomy as a second step if the first surgery is not successful.5,9,11 In a report by Spencer et al.10 for 14 patients who failed to improve after anterior callosotomy and then underwent further total callosotomy, dramatic responses were noted for all seizure types. In Korea, Kim et al.9 have reported that a second total callosotomy performed in 4 patients who did not have clinical improvement after their first surgery, allowed 3 of them to become seizure-free and allowed >75% seizure reduction in the other patient. We suggest that staged total callosotomy is a valid treatment option for patients with LGS suffering from drop attacks.
It is interesting that the EEG performed at 4 months post-operatively showed completely normal findings in our report. This finding supports the hypothesis that corpus callosum has a role in generating epileptiform discharges. Matsuo et al.12 performed a quantitative analysis of EEGs in patients who underwent corpus callosotomy; they found that corpus callosotomy decreased the total amount of spike discharge and the duration of burst activity in addition to disrupting bilateral synchrony. They suggested that corpus callosum not only transfers epileptic activities but also has a mutually facilitatory effect that enhances the epileptogenic state of both hemispheres. However, the precise role of the corpus callosum in epileptogenesis remains to be determined.
Here we report the case of a patient who experienced relapse of seizures after anterior callosotomy but finally obtained freedom from seizures with EEG normalization after the second total callosotomy.
We, therefore, suggest that although surgery can initially be limited to the anterior 2/3 of the corpus callosum, if the first operation is unsuccessful, further total callosotomy should be considered.