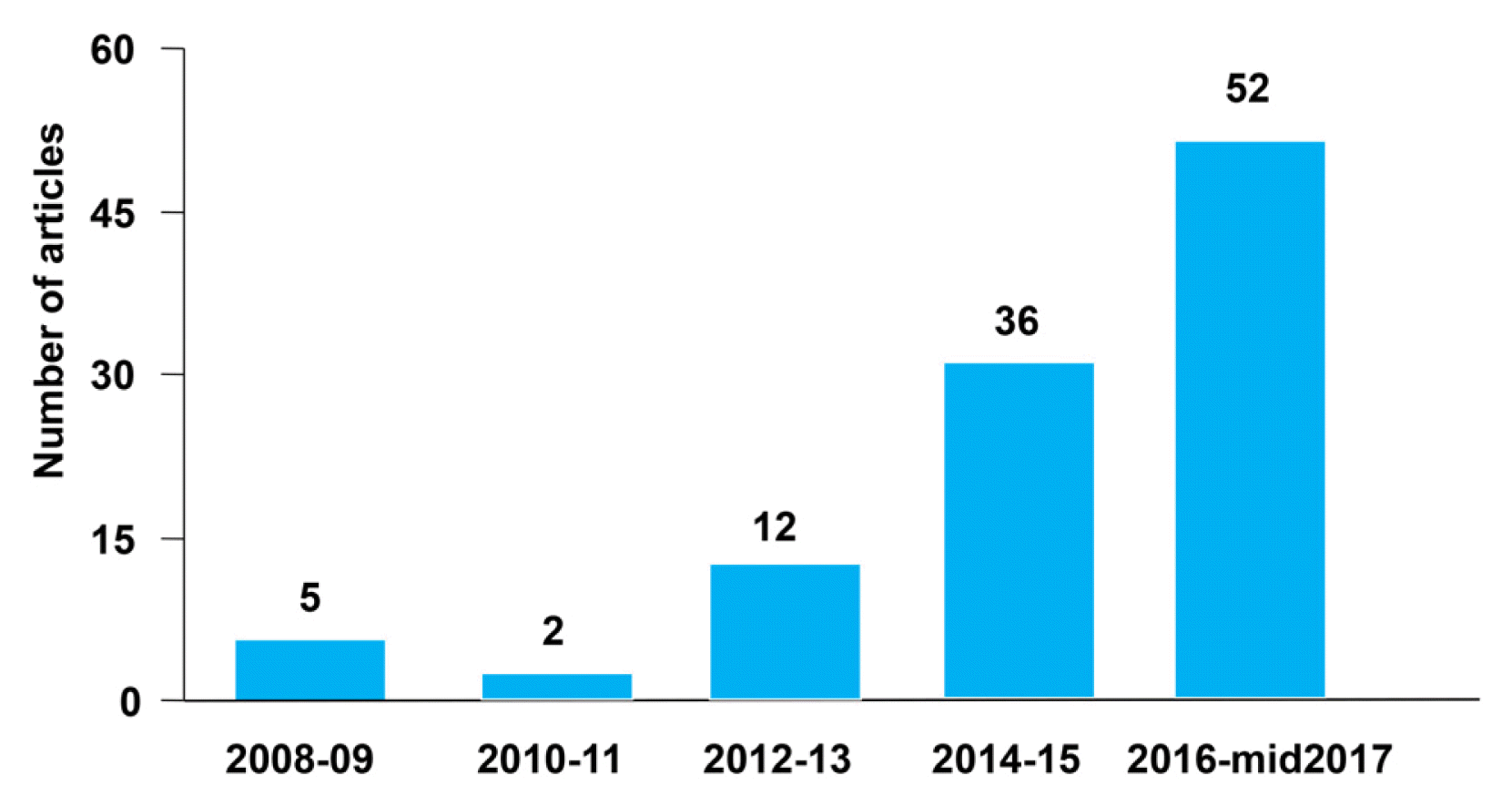
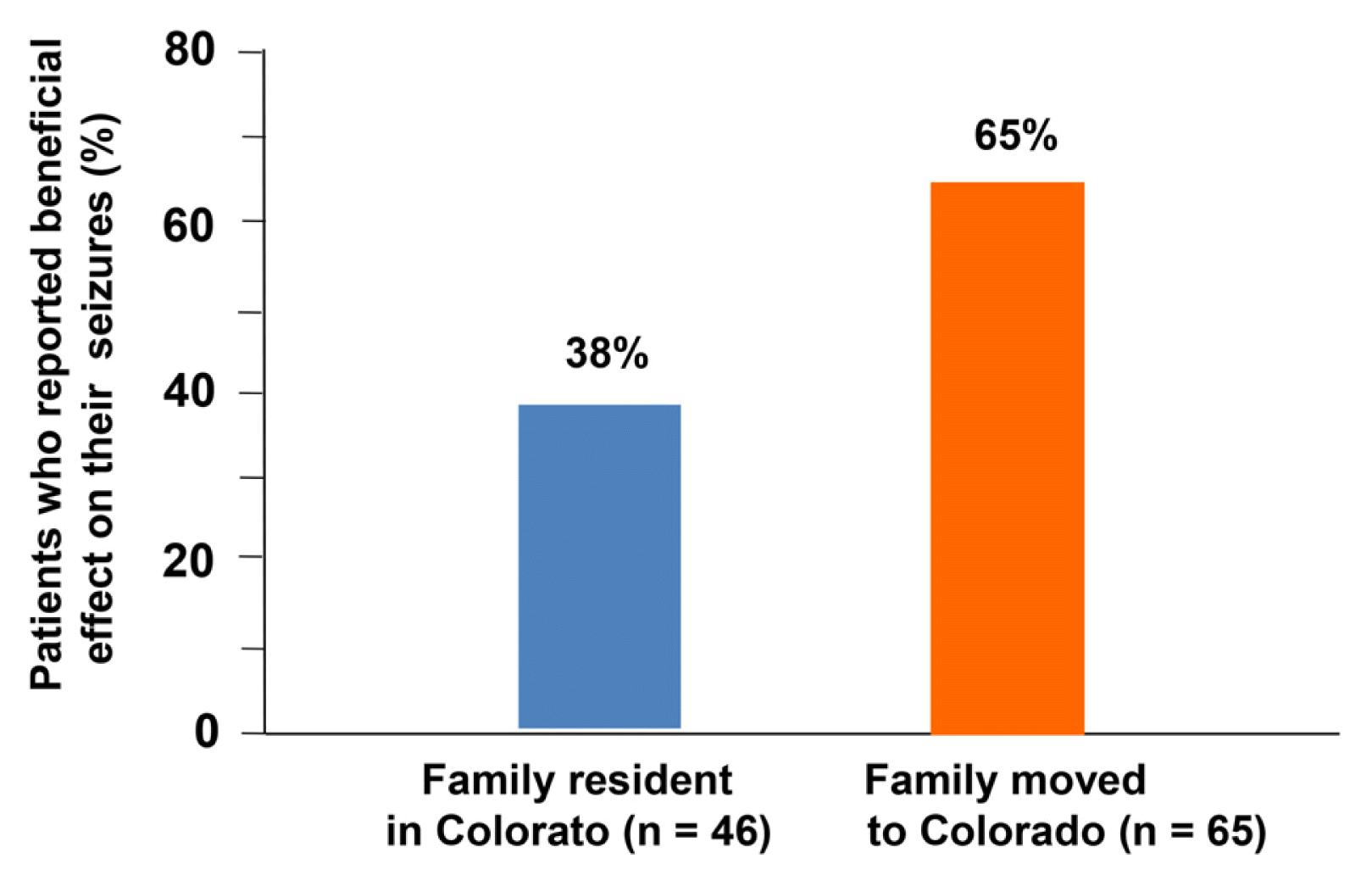1. Elsohly M. Marijuana and the Cannabinoids. Totowa: Humana Press; 2007.

2. Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav. 2017;70:Pt B. 298–301.


3. Russo EB. Cannabis and epilepsy: an ancient treatment returns to the fore. Epilepsy Behav. 2017;70:Pt B. 292–7.


5. O’Shaughnessy WB. On the preparations of the Indian hemp, or Gunjah: Cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 1843;5:363–9.

6. Gowers W. Epilepsy and other chronic convulsive disorders. London: Churchill; 1881.
7. ElSohly M, Gul W. Constituents of cannabis sativa. Petwee RG, editor. Handbook of Cannabis. Oxford: Oxford University Press; 2014. p. 3–22.

8. Chandra S, Lata H, ElSohly MA, Walker LA, Potter D. Cannabis cultivation: methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017;70:Pt B. 302–12.


9. Reddy DS, Golub VM. The pharmacological basis of cannabis therapy for epilepsy. J Pharmacol Exp Ther. 2016;357:45–55.


10. Hofmann ME, Frazier CJ. Marijuana, endocannabinoids, and epilepsy: potential and challenges for improved therapeutic intervention. Exp Neurol. 2013;244:43–50.


11. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47.


15. Lupica CR, Hu Y, Devinsky O, Hoffman AF. Cannabinoids as hippocampal network administrators. Neuropharmacology. 2017;124:25–37.


16. Katona I. Cannabis and endocannabinoid signaling in epilepsy. Handb Exp Pharmacol. 2015;231:285–316.


17. Soltesz I, Alger BE, Kano M, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–77.


18. Romigi A, Bari M, Placidi F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51:768–72.


19. Ludányi A, Eross L, Czirják S, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–90.


21. Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134:Pt 4. 1033–40.


22. Benbadis SR, Sanchez-Ramos J, Bozorg A, et al. Medical marijuana in neurology. Expert Rev Neurother. 2014;14:1453–65.


23. Detyniecki K, Hirsch L. Marijuana use in epilepsy: the myth and the reality. Curr Neurol Neurosci Rep. 2015;15:65–70.


24. Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–58.


29. Hill AJ, Jones NA, Smith I, et al. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett. 2014;566:269–74.


30. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–41.


33. Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70:Pt B. 313–8.


34. Rong C, Lee Y, Carmona NE, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213–8.


36. Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia. 2017;58:181–221.


38. Solimini R, Rotolo MC, Pichini S, Pacifici R. Neurological disorders in medical use of cannabis: an update. CNS Neurol Disord Drug Targets. 2017;16:527–33.


39. Leite JR, Carlini EA, Lander N, Mechoulam R. Anticonvulsant effects of the (−) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology. 1982;24:141–6.


40. Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur Pharmacol. 1982;83:293–8.

41. Klein BD, Jacobson CA, Metcalf CS, et al. Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42:1939–48.


43. Karler R, Turkanis SA. Cannabis and epilepsy. Adv Biosci. 1978;22–23:619–41.

44. Karler R, Turkanis SA. The cannabinoids as potential antiepileptics. J clin Pharmacol. 1981;21:8–9 Suppl. 437S–48.


45. Consroe P, Wolkin A. Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26–32.

46. Gobira PH, Vilela LR, Gonçalves BD, et al. Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology. 2015;50:116–21.


47. Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52.


48. Chiu P, Olsen DM, Borys HK, Karler R, Turkanis SA. The influence of cannabidiol and delta 9-tetrahydrocannabinol on cobalt epilepsy in rats. Epilepsia. 1979;20:365–75.


50. Turkanis SA, Smiley KA, Borys HK, Olsen DM, Karler R. An electro-physiological analysis of the anticonvulsant action of cannabidiol on limbic seizures in conscious rats. Epilepsia. 1979;20:351–63.


51. Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133:79–97.


52. Zuardi AW, Crippa JA, Hallak JE, et al. A critical review of the anti-psychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18:5131–40.


53. Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–30.


56. Mannucci C, Navarra M, Calapai F, et al. Neurological aspects of medical use of cannabidiol. CNS Neurol Disord Drug Targets. 2017;16:541–53.


59. Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. Prog Chem Org Nat Prod. 2017;103:61–101.


60. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–60.


61. Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70:Pt B. 328–33.


63. Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 2015;111:85–141.


64. Ohlsson A, Lindgren JE, Andersson S, et al. Single dose kinetics of cannabidiol in man. Agurell S, Dewey WL, Willette R, editors. The cannabinoids: chemical, pharmacologic, and therapeutic aspects. New York: Academic Press; 1984. p. 219–25.

65. Stott CG, White L, Wright S, Wilbraham D, Guy GW. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol. 2013;69:825–34.


66. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70.


67. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46:86–95.


68. Sativex. Summary of Product Characteristics (March 2015 revision) [Internet]. Newbury: GW Pharma Ltd; [cited 2017 Jul 6]. Available at: https://www.medicines.org.uk/emc/medicine/23262
.
69. Wright S, Devinsky O, Thiele EA, et al. Cannabidiol (CBD) in Dravet syndrome: a randomised, dose-ranging pharmacokinetics and safety trial (GWPCARE1). In : 32nd International Epilepsy Congress; 2017 Sep 2 – Sep 6; Barcelona, Spain Epilepsia. In press.
71. Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39:2049–56.


72. Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8.


73. Zendulka O, Dovrtělová G, Nosková K, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17:206–26.


74. Arellano AL, Papaseit E, Romaguera A, Torrens M, Farré M. Neuropsychiatric and general interactions of natural and synthetic cannabinoids with drugs of abuse and medicines. CNS Neurol Disord Drug Targets. 2017;16:554–66.


75. Reddy DS. The utility of cannabidiol in the treatment of refractory epilepsy. Clin Pharmacol Ther. 2017;101:182–4.


76. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51.


77. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP; UAB CBD Program. Interactions between cannabidiol and commonly used anti-epileptic drugs. Epilepsia. 2017;581586–92.
78. Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of Δ
9-tetrahydrocannabinol and antagonist activity of Cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–6.


79. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27.


80. Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Δ(9) -tetrahydrocannabinol in mice: implications for medical cannabis. Br J Pharmacol. 2016;173:53–65.


82. Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC. Interactions between cannabidiol and Δ
9-THC following acute and repeated dosing: rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol. 2017;27:132–45.


84. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–8.


85. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20.


86. Mazurkiewicz-Beldzinska M, Thiele EA, Benbadis S, et al. Treatment with cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a multi-centre, randomised, double-blind, placebo-controlled trial (GWPCARE4). In : 32nd International Epilepsy Congress; 2017 Sep 2 – Sep 6; Barcelona, Spain Epilepsia. In press.
87. O’Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. 2017;70:Pt B. 341–8.


88. Consroe PF, Wood GC, Buchsbaum H. Anticonvulsant nature of marihuana smoking. JAMA. 1975;234:306–7.


89. Ellison JM, Gelwan E, Ogletree J. Complex partial seizure symptoms affected by marijuana abuse. J Clin Psychiatry. 1990;51:439–40.

90. Mortati K, Dworetzky B, Devinsky O. Marijuana: an effective anti-epileptic treatment in partial epilepsy? A case report and review of the literature. Rev Neurol Dis. 2007;4:103–6.

91. Massot-Tarrús A, McLachlan RS. Marijuana use in adults admitted to a Canadian epilepsy monitoring unit. Epilepsy Behav. 2016;63:73–8.


92. Gross DW, Hamm J, Ashworth NL, Quigley D. Marijuana use and epilepsy: prevalence in patients of a tertiary care epilepsy center. Neurology. 2004;62:2095–7.


93. Brust JC, Ng SK, Hauser AW, et al. Marijuana use and the risk of new onset seizures. Trans Am Clin Climatol Assoc. 1992;103:176–81.


94. Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967;28:7 Pt 1. 474–5.

95. Hamerle M, Ghaeni L, Kowski A, Weissinger F, Holtkamp M. Cannabis and other illicit drug use in epilepsy patients. Eur J Neurol. 2014;21:167–70.


96. Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017;70:Pt B. 288–91.


97. Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313:2491–3.


98. U.S. Food and Drug Administration. 2016 Warning letters and test results for cannabidiol-related products [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; [cited 2017 Jun 26]. Available at: https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm484109.htm
.
99. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: an evidence review and research agenda. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington DC: National Academy Press; 2017.
101. Hausman-Kedem M, Kramer U. Efficacy of medical cannabis for treatment of refractory epilepsy in children and adolescents with emphasis on the Israeli experience. Isr Med Assoc J. 2017;19:76–8.

102. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55:783–6.


104. Hussain SA, Zhou R, Jacobson C, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox-Gastaut syndrome. Epilepsy Behav. 2015;47:138–41.


105. Suraev AS, Todd L, Bowen MT, et al. An Australian nationwide survey on medicinal cannabis use for epilepsy: history of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav. 2017;70:Pt B. 334–40.


107. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52.


108. Treat L, Chapman KE, Colborn KL, Knupp KG. Duration of use of oral cannabis extract in a cohort of pediatric epilepsy patients. Epilepsia. 2017;58:123–7.


109. Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. 2016;35:41–4.


110. Perucca E, Wiebe S. Not all that glitters is gold: a guide to the critical interpretation of drug trials in epilepsy. Epilepsia Open. 2016;1–2:9–21.

112. Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften. 1978;65:174–9.


113. Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–85.


114. Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. South Afr Med J. 1986;69:14
115. Trembly B, Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. In : Marijuana ‘90 International Conference on Cannabis and Cannabinoids; 1990 Jul 8–Jul 11; Kolympari, Greece.
116. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3. CD009270
118. Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia. 2017;58:e96–100.


119. Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617–24.


121. Gofshteyn JS, Wilfong A, Devinsky O, et al. Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32:35–40.


122. Kaplan EH, Offermann EA, Sievers JW, Comi AM. Cannabidiol treatment for refractory seizures in Sturge-Weber syndrome. Pediatr Neurol. 2017;71:18–23.e2.


123. Saade D, Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015;52:544–7.


124. Zuberi S, Devinsky O, Patel A, et al. Cannabidiol (CBD) significantly decreases drop and total seizure frequency in Lennox-Gastaut syndrome (LGS): Rresults of a dose-ranging, multi-centre, randomised, double-blind, placebo-controlled trial (GWPCARE3). In : 32nd International Epilepsy Congress; 2017 Sep 2 – Sep 6; Barcelona, Spain Epilepsia. In press.
125. Tamm L, Epstein JN, Lisdahl KM, et al. Impact of ADHD and cannabis use on executive functioning in young adults. Drug Alcohol Depend. 2013;133:607–14.


127. Di Forti M, Marconi A, Carra E, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–8.


128. Wolff V, Jouanjus E. Strokes are possible complications of cannabinoids use. Epilepsy Behav. 2017;70:Pt B. 355–63.


129. Crocker CE, Tibbo PG. Cannabis and the maturing brain: role in psychosis development. Clin Pharmacol Ther. 2015;97:545–7.


130. Mandelbaum DE, de la Monte SM. Adverse structural and functional effects of marijuana on the brain: evidence reviewed. Pediatr Neurol. 2017;66:12–20.


131. Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–8.


132. Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–9.


133. Hegde M, Santos-Sanchez C, Hess CP, Kabir AA, Garcia PA. Seizure exacerbation in two patients with focal epilepsy following marijuana cessation. Epilepsy Behav. 2012;25:563–6.


134. Babalonis S, Haney M, Malcolm RJ, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. 2017;172:9–13.


135. Mathern GW, Beninsig L, Nehlig A. Fewer specialists support using medical marijuana and CBD in treating epilepsy patients compared with other medical professionals and patients: result of Epilepsia’s survey. Epilepsia. 2015;56:1–6.

139. Alpár A, Di Marzo V, Harkany T. At the tip of an iceberg: prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biol Psychiatry. 2016;79:e33–45.