Although respiration difficulties are not uncommon during epileptic activity, seizures with apnea only are quite rare in adults. We report a case of a patient with seizures who presented with obstructive sleep apnoea; the seizures were documented by continuous EEG and polysomnography as well as ictal single photon emission computed tomography (SPECT).
Case report
A 20-year-old man visited our sleep center because of snoring and sleep apnoea; the symptoms had started two years prior. He worked for the military, and his apnea and snoring were bothering colleagues in the barracks. His daily life was normal, and he had neither a family history nor a medical history of seizures and had previously been healthy. His Epworth Sleepiness Scale (ESS) score was 14 out of 24, which suggested moderate excessive daytime sleepiness. He especially complained of severe sleepiness in the morning. No parasomnia events or periodic limb movements during sleep had been reported. No tongue biting was reported, but he reported recent occasional enuresis. Physical examination revealed no apparent abnormalities. His body mass index (BMI) was 23.1 kg/m2.
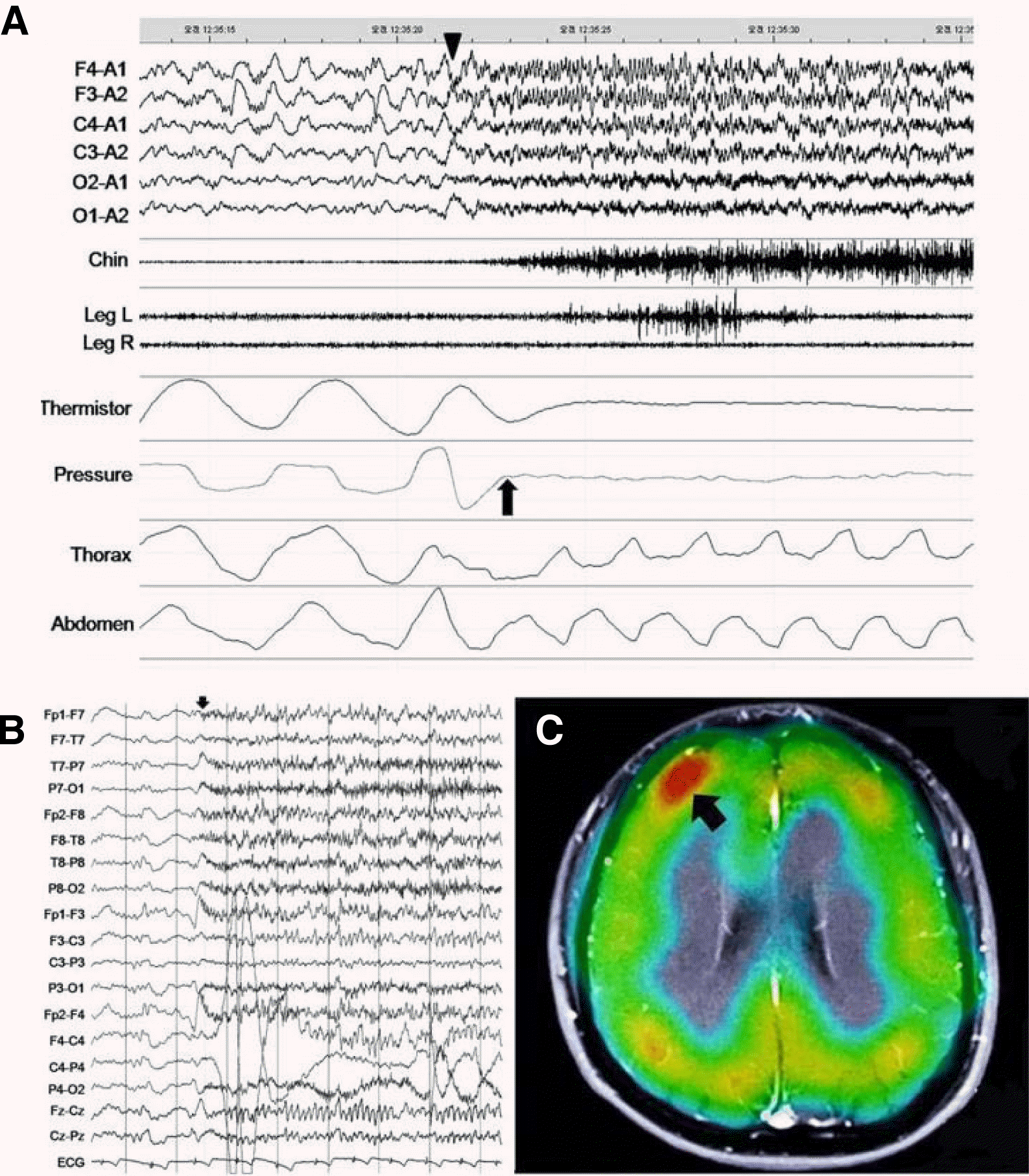
Overnight polysomnography was performed. Sleep efficiency was 81.9%, wakefulness after sleep onset (WASO) time was 59.2 minutes (17.8%), and his arousal index was 28.7/h. Stage 1 and 2 sleep were increased; however, stage 3 and REM sleep were decreased. He experienced nine apneas and two hypopneas during PSG, each episode lasted 20–40 seconds. His apnea/hypopnea index (AHI) was 2.7/h. These apneic episodes consisted of respiratory cessation accompanied by chest and abdominal movements of the obstructive type. All hypopnea was typical hypopneas. Seven out of nine apneic episodes were accompanied by rhythmic paroxysmal discharges in the bifrontal areas on EEG; clinically, five of the episodes progressed to tonic movement of all four limbs, and two episodes showed apneas only. All that seven episodes occurred during NREM sleep (two in stage 1, four in stage 2, and the others in stage 3). The other apnea and hypopnea did not accompany tonic movement.
The next night, a 16-channel video-EEG monitoring was performed to classify these events and to determine the temporal relationship of apnea onset and Ictal discharge. Ten apneic events with ictal discharges preceding the apnoea were recorded throughout the night. Ictal EEG showed paroxysmal rhythmic discharges in the range of 9 to 10Hz in the bifrontal area starting just before onset of the apneic episodes. The interictal EEG was normal, and brain MRI showed no specific abnormality. Ictal SPECT revealed increased cerebral blood perfusion in the right frontal region (Figure 1). Given these findings, we concluded that these events were epileptic seizures presenting as obstructive sleep apnoea. Carbamazepine therapy was initiated, and the nocturnal episodes were decreased.
Discussion
To our knowledge, this is the first adequately documented case report of pure epileptic seizures presenting with obstructive type sleep apnea as initial symptom. We repeatedly documented that the ictal discharges clearly preceded the sleep apnea episodes. Respiratory arrest as part of a seizure pattern was first described by Jackson.1 Apneic seizures are known to be very rare in adults. Apnea during a seizure may be due to muscle contraction of the respiratory muscles or reduced central breathing drive (central apnea). Most of the case reports regarding apneic seizures are of the central apnoea type.2,3 However, in this case, the apnoea was of the obstructive type, which may have been caused by tonic contraction of the respiratory muscles. There are just a few cases of epileptic seizure presenting as OSA that have been reported. Oldani reported two patients who showed obstructive apnea during seizures in the night.4 But the apneas were not the typical initial symptoms in those cases. In a case of insular seizures, the ictal EEG was normal, and the seizure focus was only identified using ictal SPECT, showing hyperperfusion in the left insula.5 There is also a report of an adult patient with the coexistence of sleep apnoea and tonic seizures mimicking apnoea.6 However, only interictal spikes were recorded and imaging studies were not fully described in that report, so an origin of the seizures could not be determined. Our patient is unique in that the apnoea is the first semiologic symptom, the ictal discharges started in the bifrontal areas during all recorded episodes, and the ictal SPECT revealed an abnormality in the right frontal area.
On the basis of the polysomnography, EEG, and SPECT findings, a rare form of nocturnal frontal lobe epilepsy (NFLE) was strongly considered. NFLE has a variable semiology, but apnea is very rare.7 Most observations suggest that respiratory irregularities are most likely caused by dysfunctions of the temporal lobe and limbic system.2,3,5,8 And the apneas were central type. However, this case demonstrates that NFLE can also present with choking episodes that are initially attributed to obstructive type sleep apnea. This case shows other common characters of NFLE. NFLE is predominant in males and usually begins during adolescence.9 NFLE patients experience many brief events in one night, usually in the space of a few hours.10 Video-EEG recordings of NFLE show striking stereotypy between attacks.7 Ictal EEG shows no epileptiform activity in almost 50%; in the majority of those that do show abnormalities, focal attenuation or rhythmic theta or delta is the prominent rhythm, and 10% of the abnormalities are focal fast activity like our case.
The nerve supplies to the respiratory muscles and the larynx, both sensory and motor, occur through the vagus nerve via the superior and inferior laryngeal nerve branches. The mechanism of abnormal epileptic stimulation of the laryngeal nerve is unknown.
Obstructive sleep apnea is the most common cause for presentation to sleep centers. Obstructive sleep apnea is generally caused by airway obstruction. Therefore, the primary treatment for obstructive sleep apnea focuses on maintaining the patency of the airway. Epilepsy has various clinical presentations, and it should be included in the differential diagnosis of apnea. For these reasons, it is crucial to obtain ictal confirmation showing EEG discharges preceding apneic events. A firm diagnosis is extremely important because without it one may treat with conventional obstructive sleep apnea therapy.