Introduction
The development of brain imaging techniques for evaluating patients with intractable epilepsy has allowed for the identification of various structural brain lesions that may function as epileptogenic foci, such as those due to cortical dysplasia, vascular malformations, or tumors. Cerebral calcifications are a common incidental finding, although the epileptogenicity of these structures is often underestimated. Cerebral calcifications arise from the deposition of crystalline calcium in the brain parenchyma due to either physiological or pathological conditions, such as infection or metabolic, neoplastic, vascular, congenital, developmental, or traumatic causes. Here, we report a case of intractable epilepsy arising from a solitary cerebral calcification.
Case
A 42-year-old, right-handed male was admitted to the epilepsy clinic with a history of drug-resistant seizures since the age of 18. His frequent seizures included psychic auras that often progressed to the loss of awareness and clonic movement of his left arm and leg. These episodes occurred while taking carbamazepine, valproate, and levetiracetam.
He had no previous medical history of trauma, no history of eating raw fish or meat, and no pertinent family history. His neurological examination and laboratory studies, including eosinophil count and calcium levels, were normal. Brain magnetic resonance imaging revealed a slightly high signal change in the right amygdala and a small, calcified lesion (4.5 × 4.7 mm) in the right lateral temporal region (Fig. 1). A video electroencephalography (EEG) monitoring study demonstrated frequent interictal epileptiform activity localized in the right temporal region. Ictal EEG exhibited maximum amplitude over the right frontotemporal electrodes and ictal single-photon emission computed tomography (SPECT) showed hyperperfusion in the right anterior and mesial temporal regions. Brain 18 F-fludeoxyglucose positron emission tomography (FDG-PET) revealed hypo-metabolism in the right temporal region, including the mesial temporal area. A multidisciplinary epilepsy case review panel decided to perform invasive monitoring with subdural electrodes.
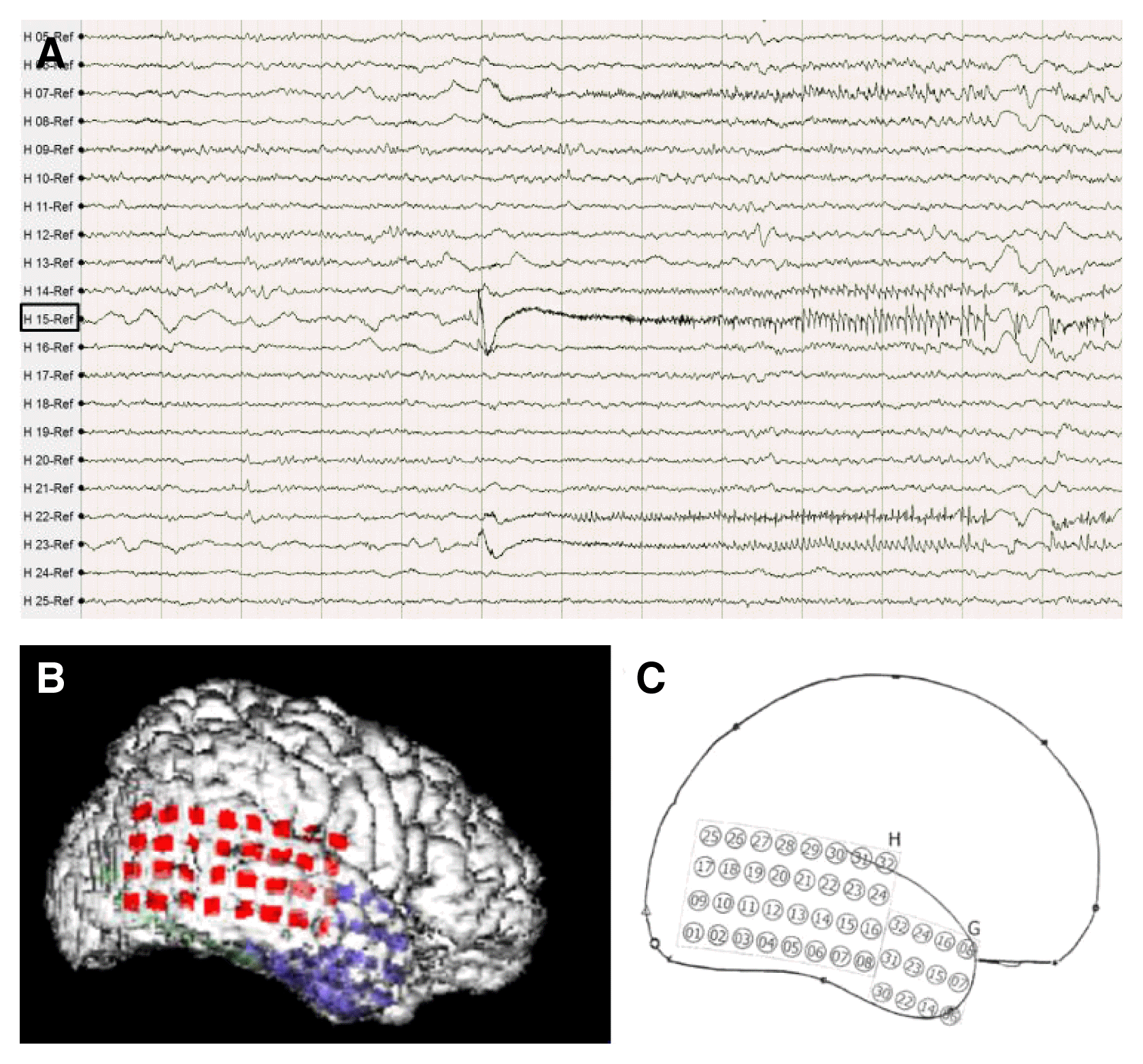
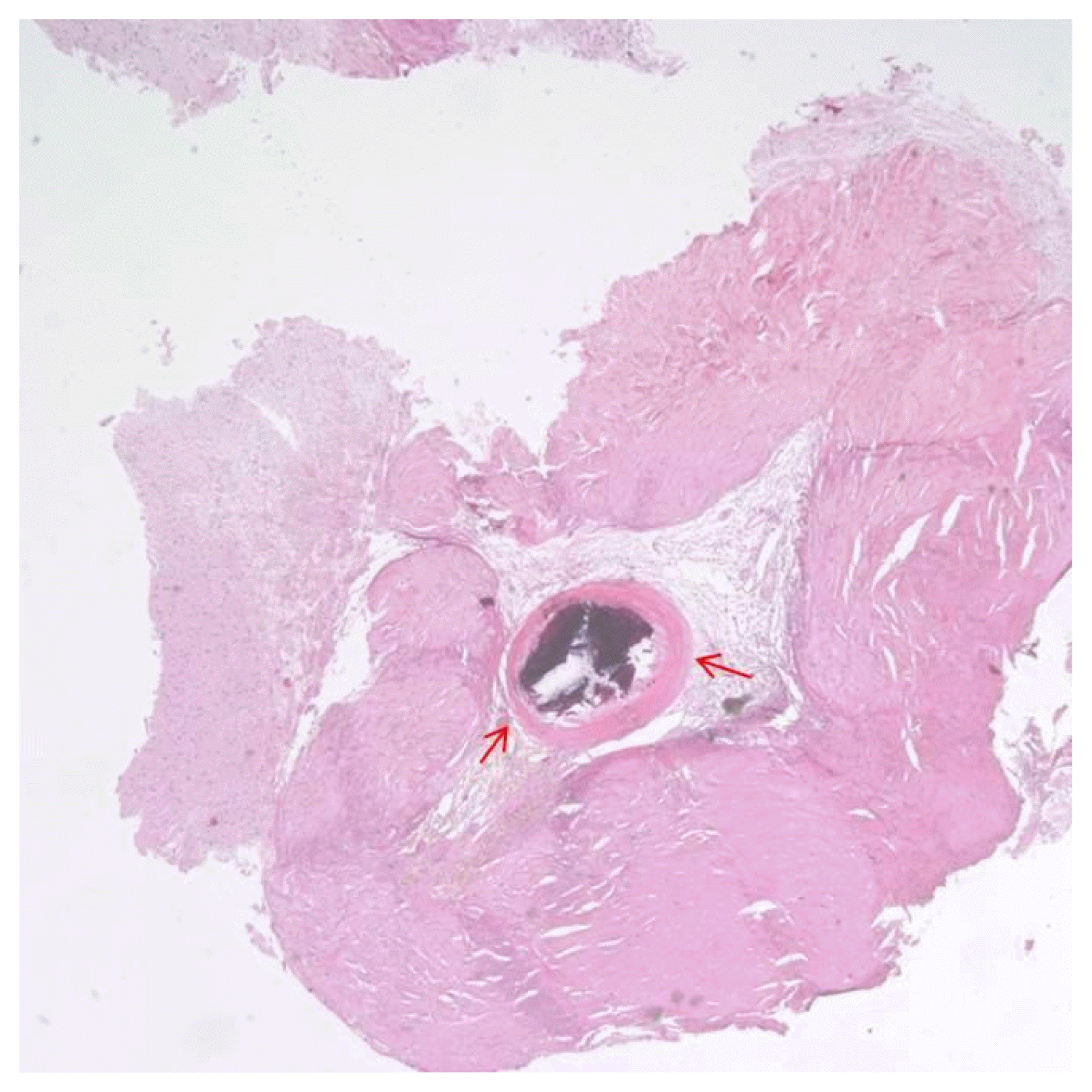
A right-sided craniotomy was performed to place a subdural electrode grid, as well as strip electrodes, over the basal, mesial, and lateral temporal regions. Platinum contact electrodes embedded in 0.5-mm-thick flexible silicone plates (AD-Tech Medical Instrument Corp, Racine, WI, USA) were used. A 64-channel video EEG recording device was used to obtain the measurements. Four days after electrode placement, the patient had five habitual seizures with automatisms featuring right hand fumbling but no aura. During these seizures, fast activity was observed to initiate in the right lateral temporal area just above the solitary calcified lesion (Fig. 2B, C: electrode H15). From that point, activity spread into the basal region and then the entire lateral temporal region (Fig. 2). Five days after electrode placement, subclinical seizures initiating above the mesiobasal temporal lesion and spreading over the temporal region were detected via EEG. Six days after electrode placement, the patient underwent an anterior temporal lobectomy with partial amygdalohippocampectomy. Histological examination of the small, calcified lesion showed no specific findings apart from being a fibrocalcific nodule (Fig. 3). Examination of the amygdala showed deposits of corpora amylacea, but the hippocampus showed no abnormalities. The patient experienced no further events and has been seizure-free for 24 months without antiepileptic drugs.
Discussion
This report describes a patient with intractable epilepsy arising from a solitary cerebral calcification. Unfortunately, it was not possible to determine the etiology of this lesion, as the neuropathological findings were inconclusive apart from the presence of a fibrocalcific nodule.
The identification of focal lesions is particularly important when patients with medically refractory partial seizures are considered for epilepsy surgery. Indeed, the best outcomes after surgery are observed when focal structural lesions are identified and are concordant with EEG findings. In a previous neuropathological report, the most common causes of intractable epilepsy were focal cortical dysplasia, scar lesions due to hypoxia/ischemia or trauma, and brain tumors. Cerebral infections such as neurocysticercosis and tuberculous meningitis were not commonly observed, except in endemic areas.1 Single-lesion neurocysticercosis might cause seizure recurrence in 14.5% of patients.2
A solitary cerebral calcification is defined as a single small (< 10 mm) calcified lesion.3 The most common causes of these lesions are cerebral infections or their sequelae, including cysticercosis, tuberculomas, or fungal granulomas. Other causes of solitary cerebral calcifications include low-grade calcifying tumors, such as oligodendrogliomas, gangliogliomas, or dysembryoplastic neuroepithelial tumors,4 and vascular lesions such as small arteriovenous malformations.3,5,6 In this case, a neuropathological analysis of the lesion revealed no other primary disease.
Patients with solitary cerebral calcification-related epilepsy may show varying courses, from simple to intractable seizures. In a previous imaging study,7,8 gliosis or edematous changes around a cerebral calcified lesion and persistent enhancement of the lesion predicted poor seizure control. Poor seizure control was due to the development of perilesional pathognomonic changes due to inflammatory aggravation caused by an underlying disease9 or recurrent seizures. EEG abnormalities, a family history of epilepsy, and serial seizures were found to be additional risk factors for seizure recurrence.9
In this case, the patient showed a subclinical seizure arising from the mesiobasal temporal region. Brain SPECT and FDG-PET also suggested defects in the mesiobasal temporal area, and neuro-pathological assessment of the amygdala revealed deposits of corpora amylacea. Epilepsy due to solitary cerebral calcification may arise not only from the calcification itself but also from the adjacent mesial temporal cortex, perhaps representing a secondary kindling phenomenon. In a previous study examining the co-occurrence of calcified cysticerci and hippocampal sclerosis, about 8% of calcifications were located in the temporal lobe, ipsilateral to the hippocampal sclerosis.10,11
Thus, although solitary cerebral calcifications may be an asymptomatic or coincidental finding in some patients, they may also function as an important epileptogenic focus. Therefore, focal cortical calcification should be carefully observed in patients with intractable epilepsy.