Introduction
Many clinical conditions mimic seizure, including syncope, hypoglycemia, hemi-facial spasm, non-epileptic myoclonus, transient ischemic attack, and panic disorder.1 Sometimes these conditions are difficult to differentiate from epileptic convulsion, and they can easily lead to misdiagnoses. In cases of non-convulsive status epilepticus (NCSE), diagnosis is even more challenging because symptoms and signs are variable, subtle, and ill-defined.2 Electroencephalography (EEG) is also nonspecific in many cases. Misdiagnosis can lead to serious problems including undue delays in appropriate treatment. Herein, we describe the case of a patient with symptoms mimicking non-convulsive occipital lobe status epilepticus that were associated with hydrocephalus after traumatic hemorrhage.
Case
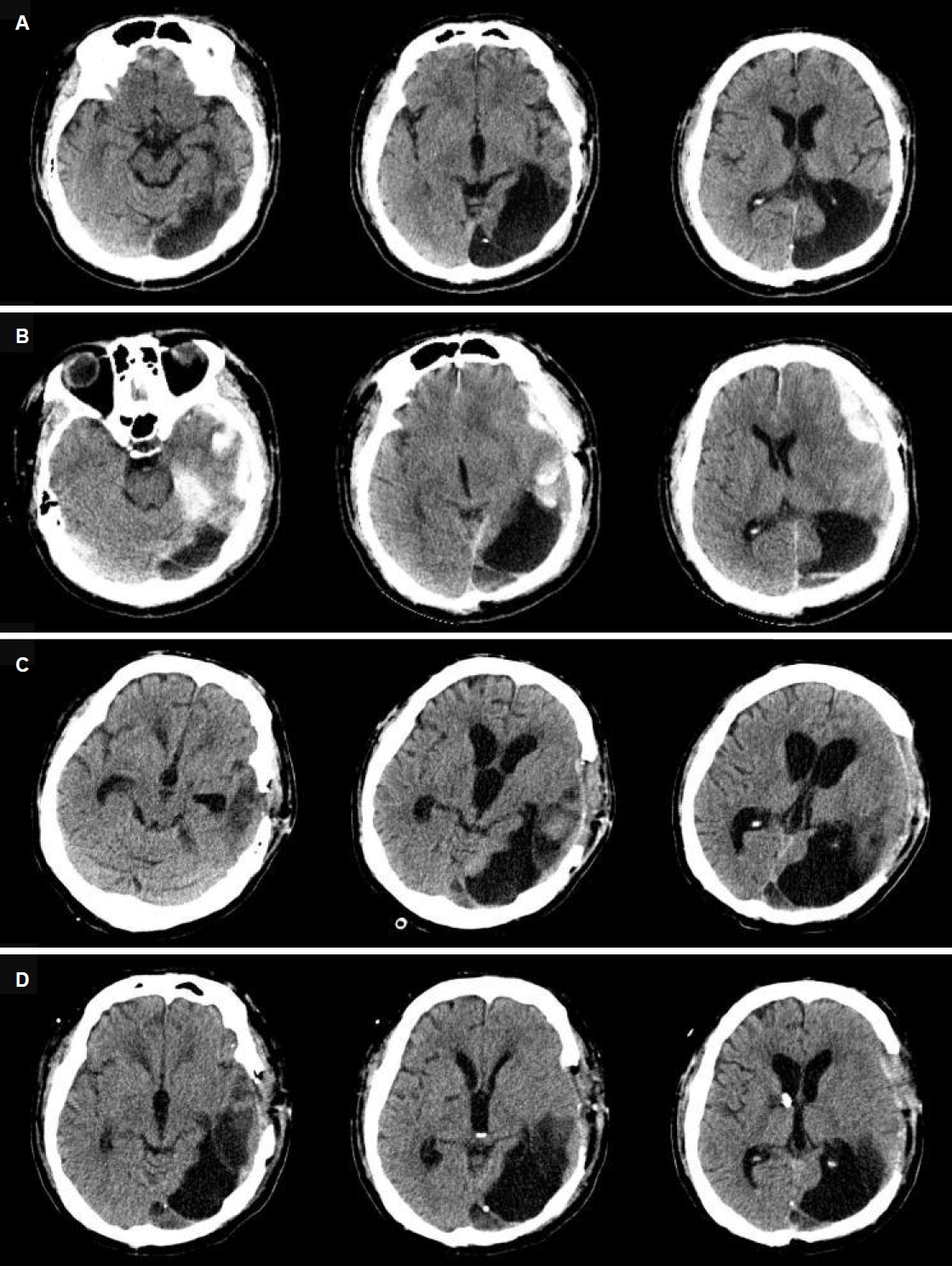
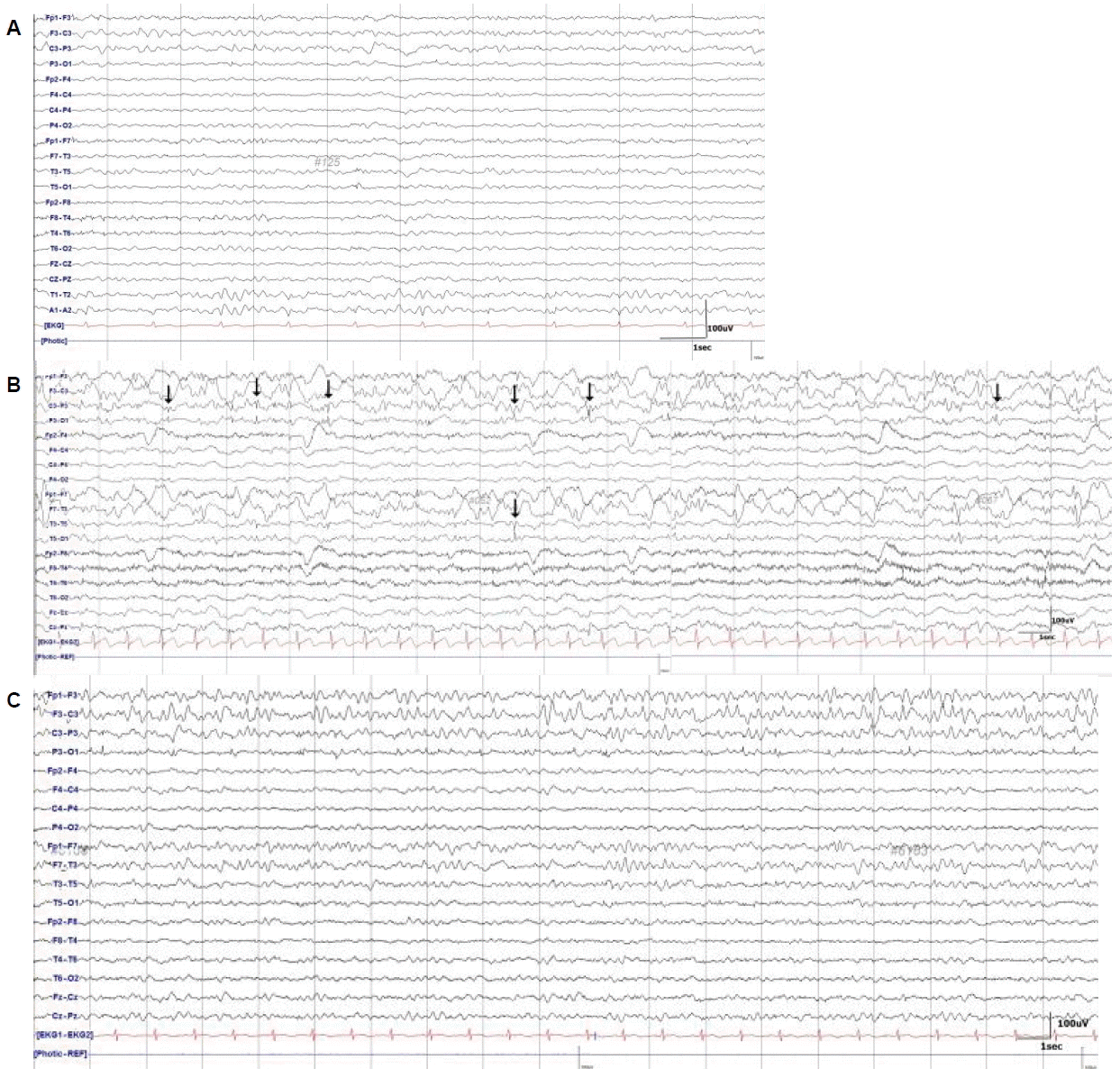
A 41-year-old man collapsed on the street and was found unconscious. He had a history of left occipital lobectomy at the age of 22 years due to intractable occipital lobe epilepsy (OLE). He was taking multiple antiepileptic drugs (AEDs) including levetiracetam 2,000 mg/day, valproic acid 500 mg/day, and carbamazepine 200 mg/day. Previous brain computed tomography (CT) revealed encephalomalacia of the left parieto-occipital lobe (Fig. 1A), and EEG revealed focal slowing in the left centro-parieto-temporal region (Fig. 2A). Despite having undergone occipital lobectomy, he had more than 5 recurrent seizure episodes within a year. In the emergency room, brain CT revealed acute subdural hemorrhage in the left frontal lobe and intraparenchymal hemorrhage in the left temporo-occipital lobe (Fig. 1B). Emergent decompressive craniectomy was performed to remove the subdural hemorrhage, but the intraparenchymal hemorrhage in the left temporo-occipital lobe remained. After the surgery, he regained consciousness and was able to perform wheelchair ambulation. The Glasgow coma scale (GCS) score at this point was 14 (eye 4, verbal 4, motor 6). However, 4 days after the surgery he became less responsive and had frequent episodes of unconsciousness accompanied by tonic upward deviations of the eyeballs lasting a few seconds. The GCS score was 9 (eye 2, verbal 3, motor 4) and abrupt mental drowsiness continued for more than 30 minutes. We initially considered this condition to be a result of non-convulsive occipital lobe status epilepticus, because he was being treated for OLE and there was an associated high possibility of exacerbation due to hemorrhage. Scalp EEG revealed frequent spikes or sharp waves in the left temporo-parietal lobe, and occasional frontal spikes and sharp waves suggesting ongoing seizure caused by hemorrhage (Fig. 2B). Intravenous lorazepam was injected at the time of the episodes, and simultaneously a modified AED regimen was administered consisting of levetiracetam from 2,000 mg/day to 3,000 mg/day, valproic acid from 500 mg/day to 1,000 mg/day, phenytoin from 200 mg/day to 300 mg/day, lamotrigine 400 mg/day, lacosamide 100 mg/day, and carbamazepine 400 mg/day. Although scalp EEG ceased to depict spikes and sharp waves (Fig. 2C), his GCS score worsened to 7 (eye 2, verbal 2, motor 3) and he exhibited virtually continuous tonic upward eyeball deviation.
Because there was no clinical improvement, further evaluation was necessary to investigate alternative causes of tonic upward eyeball deviation and mental state. Follow-up brain CT revealed bilateral enlargement of lateral ventricles, temporal horns, and the third ventricle suggesting obstructive hydrocephalus (Fig. 1C) and associated midbrain compression. To alleviate hydrocephalus, external ventricular drainage was performed followed by a ventriculo-peritoneal shunt operation (Fig. 1D). Tonic upward eyeball deviation ceased to occur, and the patient recovered consciousness. The GCS score at this point was 13 (eye 3, verbal 4, motor 6). Thereafter, doses of AEDs were reduced and adjusted to levetiracetam 1,000 mg/day, phenobarbital 60 mg/day, phenytoin 400 mg/day, and lamotrigine 400 mg/day. The patient was transferred to the rehabilitation department for active rehabilitation, and there were no recurrences of tonic upward eyeball deviation.
Discussion
In cases of NCSE, symptoms can range from mild confusion to coma.3 Thus, it is important to distinguish NCSE from other pathological conditions that causea confused mental state. Unconscious patients hospitalized in intensive care units due to head injury or other medical conditions are often not diagnosed with NCSE due to a lack of definite visible ictal symptoms.4 It is also very difficult to differentiate between the progression of underlying disease and ineffective treatment of NCSE in cases of combined serious medical or surgical conditions. Recently, we encountered an impending case mimicking non-convulsive occipital lobe status epilepticus that was induced by hydrocephalus after intracranial hemorrhage.
The typical symptoms of OLE are visual auras, visual hallucinations, ictal blindness, eye movement sensations, blinking, and eyelid fluttering.5 Paroxysmal tonic deviation of the eyeball is also a characteristic symptom of OLE. Ictal manifestations of OLE depend on the spread of epileptic discharge, and diagnosing it can be challenging because well-localized unifocal rhythmic ictal discharge is infrequent and rapid ictal spread to other brain areas.5 Surface ictal scalp EEG in patients with OLE often reveals posterior temporal activity and diffuse posterior sharp waves or spike activity with a wide field of distribution.6 The most common etiologic factor of OLE is prenatal or neoplastic lesion, and head trauma is the second most common.7
In the present case, the patient exhibited tonic upward eyeball deviations in conjunction with unconsciousness. Initially our diagnosis was non-convulsive occipital lobe status epilepticus, because he had a previous history of recurrent seizures after occipital lobectomy, and he had also recently developed an intracranial hemorrhage―a condition that has the potential to increase seizure activity. EEG improvement without clinical improvement was concordant with a possible diagnosis of NCSE according to the most recent International League Against Epilepsy description and classification of NCSE.8 Based on the results of a series of scalp EEGs and follow-up brain CTs, we surmised that the tonic upward eyeball deviation originated from structural lesion rather than epilepsy. The mesodiencephalic junction is the critical site of upward gaze control, because the anatomical structures mediating vertical-gaze input to the oculomotor nuclei are all clustered around the Sylvian aqueduct.9 Any structural lesions directly or indirectly irritating this area including stroke, vasculitis, or tumor could cause involuntary eyeball movement. We could not completely rule out the possibility of NSCE, which may have developed due to irritation of mesencephalic areas by chemical components that emerged after intracranial hemorrhage. However, it is reasonable to conclude that tonic upward eyeball deviation was induced by compression of mesodiencephalic brain due to hydrocephalus development after intracranial hemorrhage. Fortunately, via emergent surgery we were able to prevent serious conditions such as brain herniation and death.
In conclusion, tonic upward eyeball deviation may occur in non-convulsive occipital lobe status epilepticus patients, but structural lesions may induce similar signs and symptoms. Clinicians should be aware of seizure-mimicking medical or surgical conditions that are not true seizures, especially in NCSE patients, to prevent grave prognoses.