1. Edlow AG, Edlow BL, Edlow JA. Diagnosis of acute neurologic emergencies in pregnant and postpartum women. Emerg Med Clin North Am. 2016;34:943–65.

2. Edlow JA, Caplan LR, O’Brien K, Tibbles CD. Diagnosis of acute neurological emergencies in pregnant and post-partum women. Lancet Neurol. 2013;12:175–85.


3. Aya AG, Ondze B, Ripart J, Cuvillon P. Seizures in the peripartum period: epidemiology, diagnosis and management. Anaesth Crit Care Pain Med. 2016;35:Suppl 1. S13–21.


4. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–5.


5. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–30.


6. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus--report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23.


7. Leitinger M, Beniczky S, Rohracher A, et al. Salzburg consensus criteria for non-convulsive status epilepticus--approach to clinical application. Epilepsy Behav. 2015;49:158–63.


8. Haut SR. Seizure clustering. Epilepsy Behav. 2006;8:50–5.


9. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4.


10. Romano M, Cacciatore A, Giordano R, La Rosa B. Postpartum period: three distinct but continuous phases. J Prenat Med. 2010;4:22–5.


11. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–45.


12. Santos VM, Correa FG, Modesto FR, Moutella PR. Late-onset postpartum eclampsia: still a diagnostic dilemma? Hong Kong Med J. 2008;14:60–3.

13. Magee L, von Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2013;4:CD004351
14. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470–5.


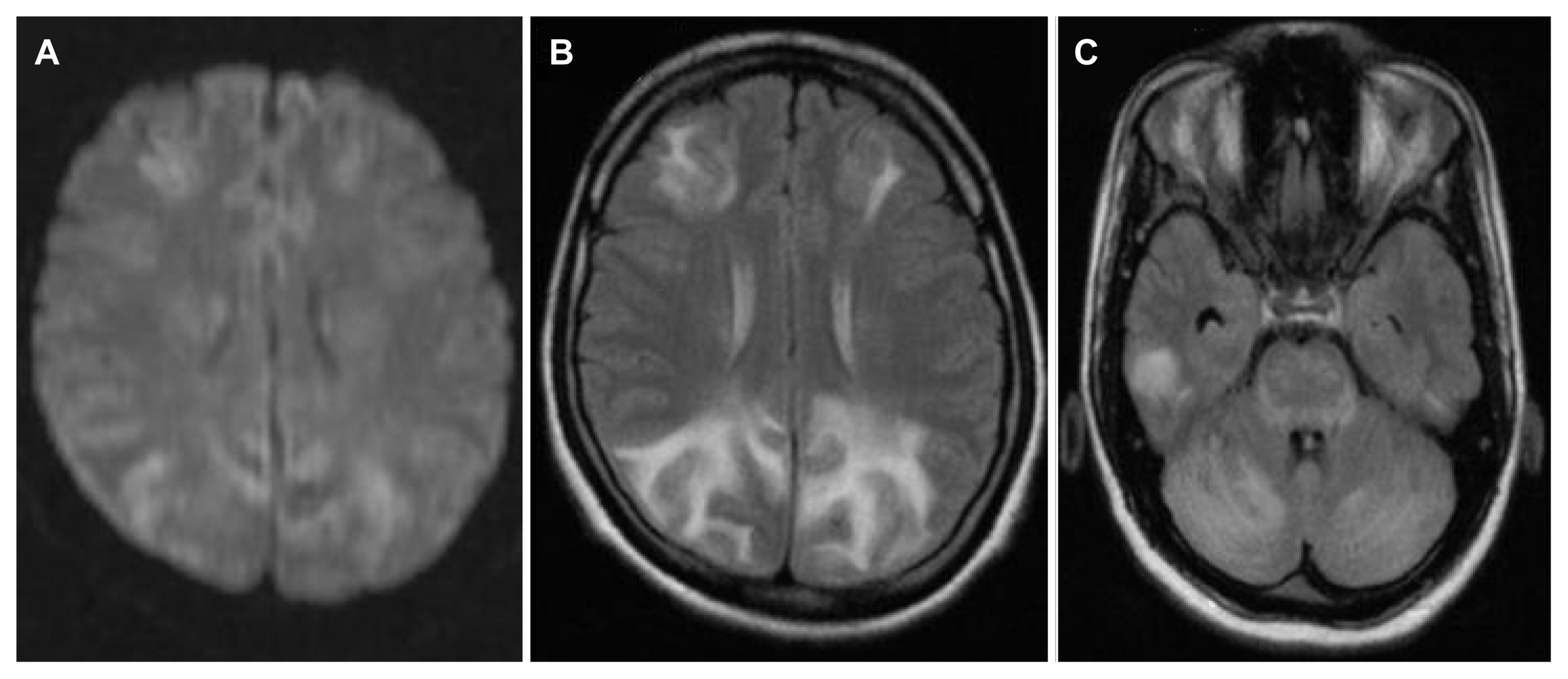
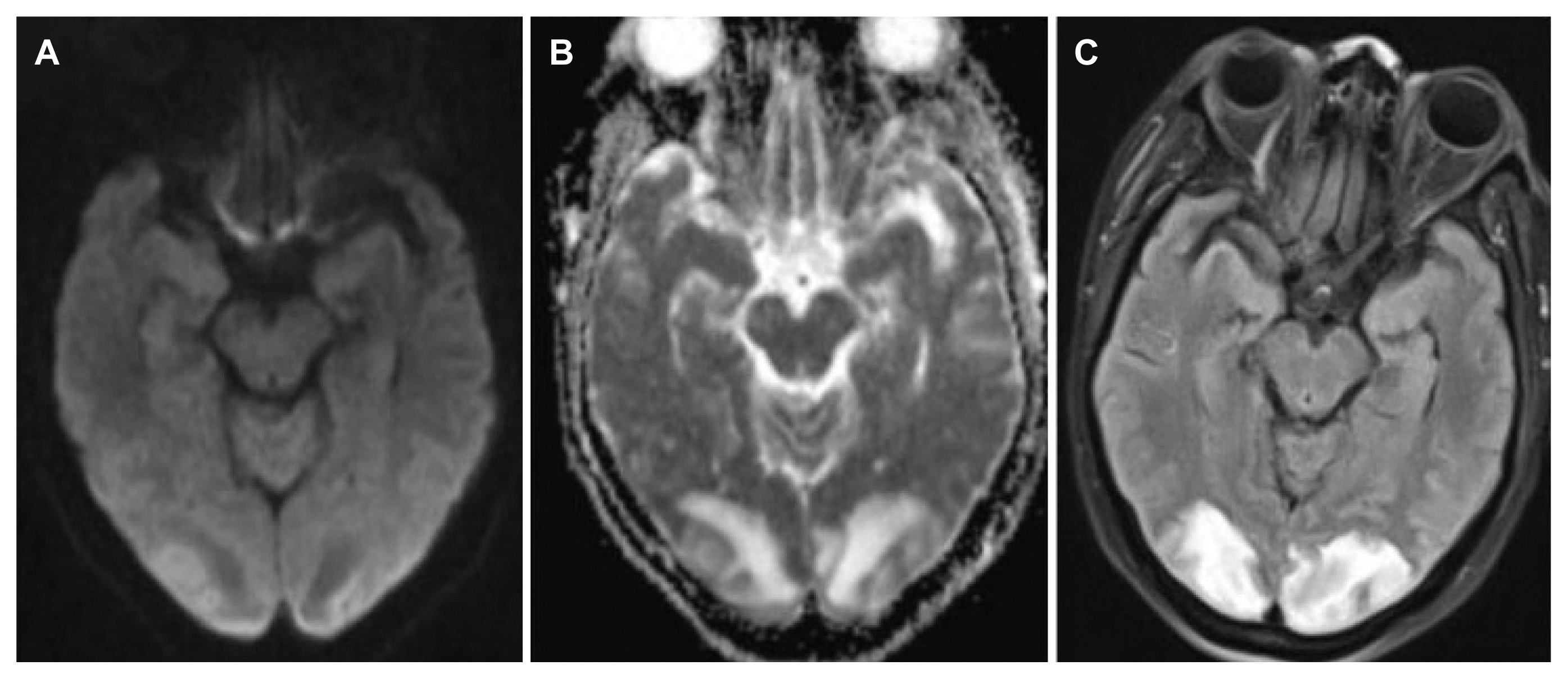
15. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.


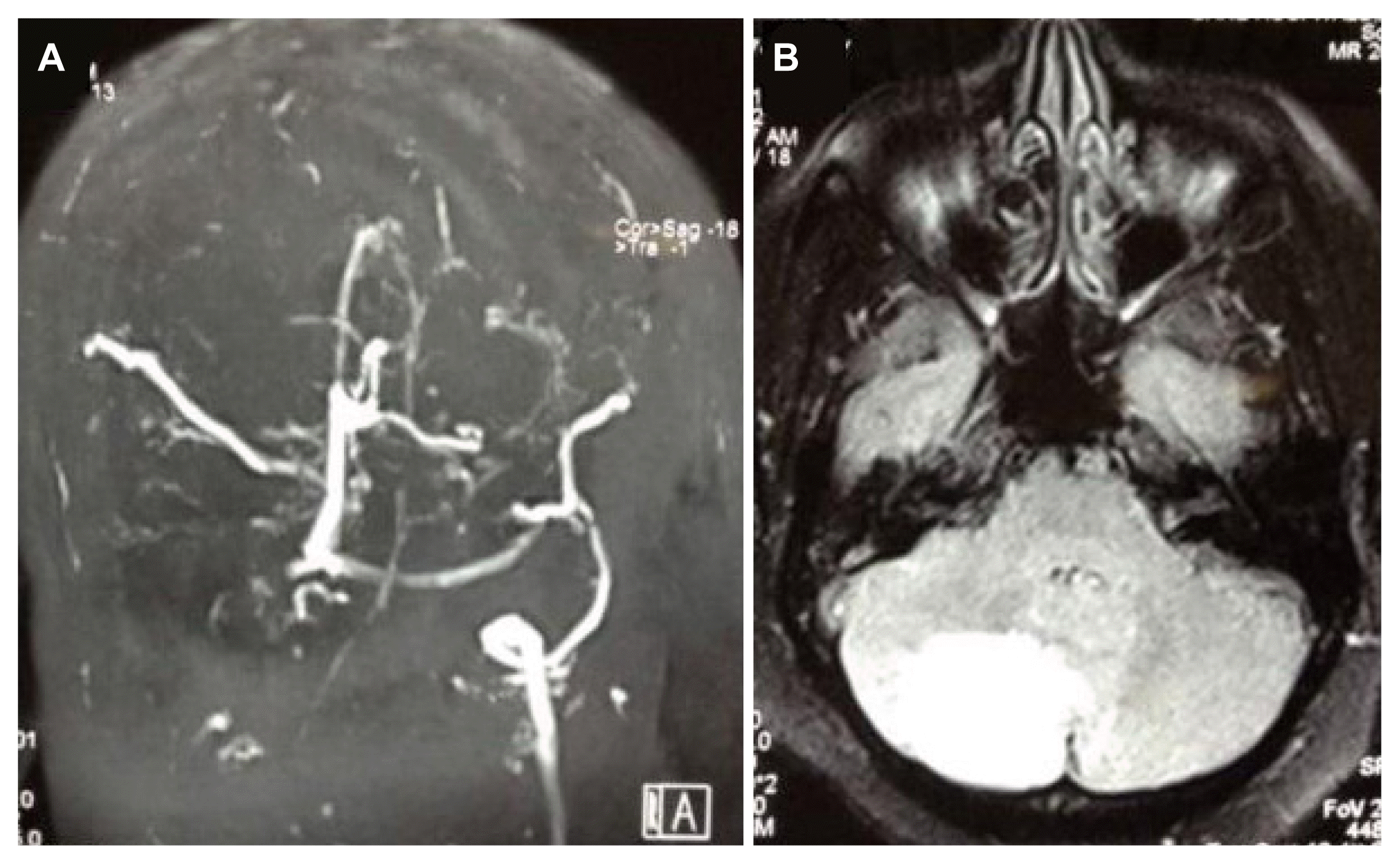
17. Narayan D, Kaul S, Ravishankar K, et al. Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: insights from Nizam’s institute venous stroke registry, Hyderabad (India). Neurol India. 2012;60:154–9.


18. Ferro JM, Canhão P, Stam J, Bousser MG. Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–70.


19. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10.


20. Shankar J, Banfield J. Posterior reversible encephalopathy syndrome: a review. Can Assoc Radiol J. 2017;68:147–53.

21. Garg RK, Kumar N, Malhotra HS. Posterior reversible encephalopathy syndrome in eclampsia. Neurol India. 2018;66:1316–23.


22. Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry. 2010;81:773–7.


24. Kang E, Sugarman R, Ramadan H, et al. Prevalence, risk factors and associated complications of postpartum hypertension in rural Haiti. Pregnancy Hypertens. 2017;10:135–42.


25. August P, Malha L. Postpartum hypertension: “It Ain’t Over ‘til It’s Over”. Circulation. 2015;132:1690–2.


26. Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19:935–43.


27. Danhofer P, Tomečková M, Černá , et al. Prognostic factors and seizure outcome in posterior reversible encephalopathy syndrome (PRES) in children with hematological malignancies and bone marrow failure: a retrospective monocentric study. Seizure. 2019;72:1–10.