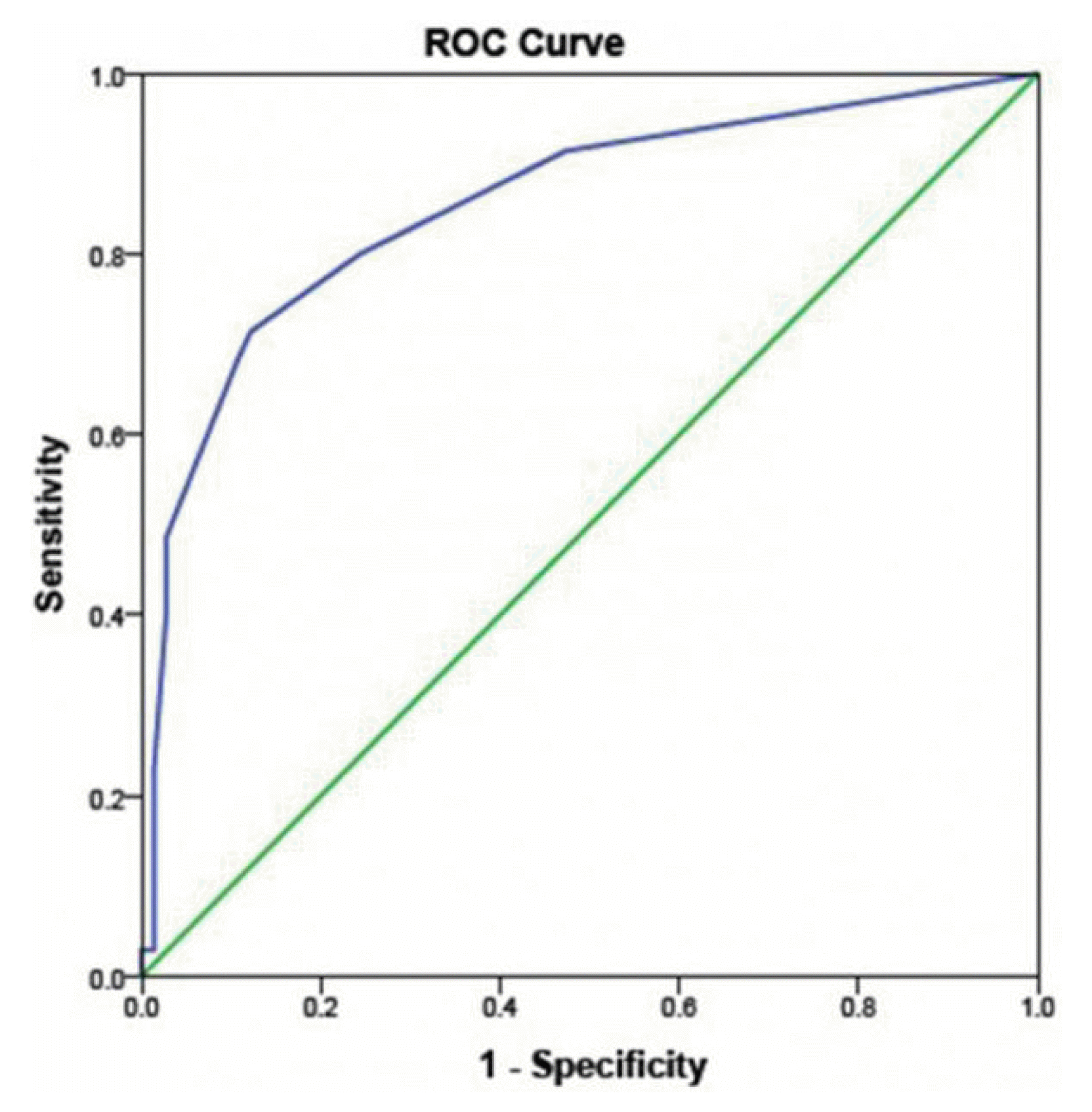
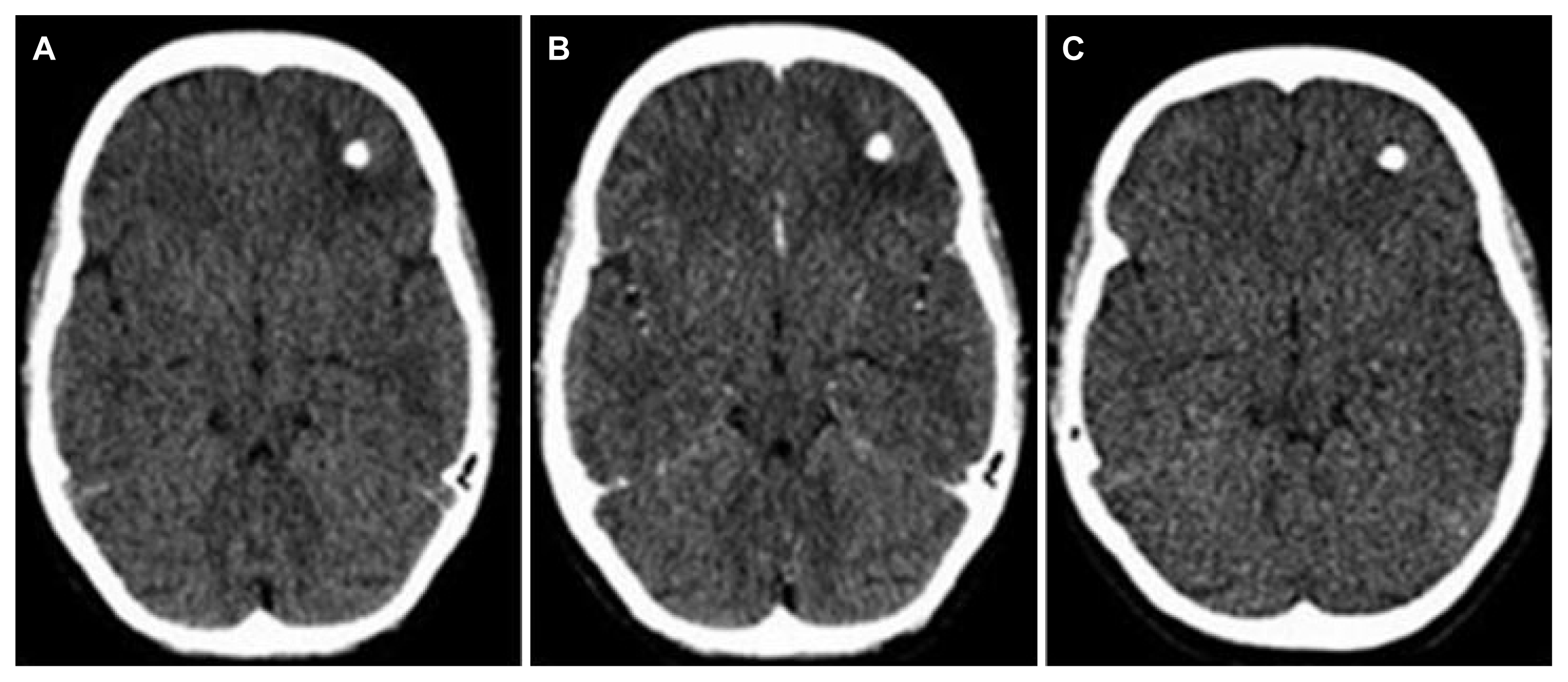
1. Commission on Tropical Diseases of the International League Against Epilepsy. Relationship between epilepsy and tropical diseases. Epilepsia. 1994;35:89–93.


2. Garcia HH, Del Brutto OH. Cysticercosis Working Group in Peru. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–61.

3. Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8.


5. Sharma LN, Garg RK, Verma R, Singh MK, Malhotra HS. Seizure recurrence in patients with solitary cystic granuloma or single parenchymal cerebral calcification: a comparative evaluation. Seizure. 2013;22:840–5.


6. Nash TE, Mahanty S, Loeb JA, et al. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia. 2015;56:177–83.


8. Gupta RK, Awasthi R, Rathore RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–25.


10. Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr. 1994;52:289–94.


11. Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. 2013;54:1815–22.


12. Del Brutto OH, Rajshekhar V, White AC, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83.


13. Blume WT, Lüders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–8.


15. Uribe J, Olvera JE. Cysticercosis human: a clinical and pathological study of 481 autopsy patients. Patologia. 1988;26:149–56.
17. Verma A, Misra S. Outcome of short-term antiepileptic treatment in patients with solitary cerebral cysticercus granuloma. Acta Neurol Scand. 2006;113:174–7.


18. Singh AK, Garg RK, Rizvi I, Malhotra HS, Kumar N, Gupta RK. Clinical and neuroimaging predictors of seizure recurrence in solitary calcified neurocysticercosis: a prospective observational study. Epilepsy Res. 2017;137:78–83.


19. Bittencourt PR, Costa AJ, Oliveira TV, Gracia CM, Gorz AM, Mazer S. Clinical, radiological and cerebrospinal fluid presentation of neurocysticercosis: a prospective study. Arq Neuropsiquiatr. 1990;48:286–95.


20. Carpio A, Placencia M, Santillán F, Escobar A. A proposal for classification of neurocysticercosis. Can J Neurol Sci. 1994;21:43–7.


21. Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med. 1985;145:442–5.


22. Wirrell EC. Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. J Clin Neurophysiol. 2010;27:239–48.


23. Mehta S, Singh G. Electroclinical characteristics of new-onset seizures associated with neurocysticercosis. Neurol India. 2014;62:159–63.


24. Nash TE, Bustos JA, Garcia HH. Cysticercosis Working Group in Perú. Disease centered around calcified taenia solium granuloma. Trends Parasitol. 2017;33:65–73.


25. Gupta RK, Kumar R, Chawla S, Pradhan S. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia. 2002;43:1502–8.


26. Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol. 2000;48:181–7.