Introduction
Epilepsy is a common chronic neurological disorder, with an estimated global prevalence of 3.3 per 1,000 population in 2016.
1 In South Korea, the estimated prevalence of treated epilepsy was 3.84 per 1,000 population across all age groups.
2 The use of anti-seizure medication (ASM) therapy is generally recommended after a second epileptic seizure, with the selection of the ASM being tailored based on the epilepsy syndrome.
3 Drug treatment usually starts with mono-therapy and when the first monotherapy fails to provide seizure control, switching to a second ASM monotherapy or add-on therapy should be considered.
3,
4 Perampanel, a selective and non-competitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptor antagonist, is a once-daily oral ASM. It is used in the treatment of focal-onset seizures (FOS; previously referred to as partial-onset seizures),
5 and as an adjunctive treatment of generalized tonic-clonic seizures (GTCS; previously referred to as primary GTCS).
6 Currently, perampanel is approved in Korea as monotherapy and adjunctive therapy for FOS with or without focal to bilateral tonic-clonic seizures (FBTCS; previously referred to as secondarily GTCS) in patients with focal epilepsy aged >4 years, and as adjunctive therapy for primary GTCS in patients with idiopathic generalized epilepsy aged >7 years.
7
The incidence of treatment-emergent adverse events (TEAEs) such as dizziness has been reported to increase with increasing per-ampanel doses,
8 and doses lower than the maximum recommended 8–12 mg/day may be better tolerated.
9–
12 Observational studies suggest that a high number of previous ASMs may be a poor prognostic factor for treatment response, with the likelihood of achieving seizure freedom significantly reducing with the number of previous ASM regimens.
13–
15 This has been shown in real-world studies of add-on perampanel, with more patients being seizure-free at 12 months when perampanel was the first add-on ASM versus the second add-on ASM
16 and better clinical responses to perampanel in patients who had received relatively few prior ASMs.
17
The FAME (Fycompa
® as first Add-on to Monotherapy in patients with Epilepsy; NCT02726074) study was a single-arm, multicenter, open-label, phase IV study conducted in South Korea.
18 FAME investigated the efficacy and safety of perampanel as the first add-on therapy in 85 patients aged ≥12 years with FOS with or without FBTCS, in whom the one or two prior ASM monotherapies were not successful in controlling seizures. Perampanel was effective as the first add-on therapy in FAME, with 50%, 75%, and 100% responder rates of 80.0%, 71.8%, and 47.1%, respectively, and was well tolerated.
18
To further characterize the clinical profile of add-on perampanel in patients with FOS, we conducted post hoc analyses to evaluate its efficacy and tolerability in additional subgroups of patients from FAME. Relevant to our interest in improving patient outcomes, we evaluated whether the efficacy and tolerability of add-on perampanel were affected by low-dose versus high-dose maintenance perampanel, per-ampanel added to first- versus second-line ASM monotherapy, and concomitant background ASM monotherapy. These results, taken together with those of primary and other post hoc analyses of FAME, may provide a more complete clinical profile of add-on perampanel in FOS that may better aid clinicians in treating patients with FOS.
Methods
Detailed methods of the FAME study are described elsewhere.
18 Briefly, the study comprised an 8-week screening period, a 12-week titration period, and a 24-week maintenance period. During the titration period, the perampanel dose was increased from 2 mg once daily at bedtime up to a maximum dose of 12 mg/day by 2 mg/day increments over the weekly intervals for 2 weeks. If intolerable TEAEs occurred, dose titration was prohibited until the TEAEs were resolved. Patients who did not tolerate perampanel 4 mg/day were excluded from the study. During the maintenance period, patients received the same dose as administered at the end of the titration period, although doses could be increased to a maximum of 12 mg/day or decreased to a minimum of 4 mg/day, depending on clinical response and tolerability.
The primary efficacy endpoint was the 50% responder rate (defined as the proportion of patients with a ≥50% reduction from baseline in total seizure frequency during the maintenance period). Secondary endpoints included 75% and 100% (seizure-free) responder rates, and the median percent change from baseline total seizure frequency per 28 days during the maintenance period. Efficacy analyses were conducted using the full analysis set (FAS; defined as patients who received at least one dose of perampanel and were included in at least one efficacy assessment). Safety endpoints were assessed using the safety analysis set (SAS; defined as patients who received at least one dose of perampanel and were included in at least one safety assessment) and included TEAE, serious TEAE, and treatment withdrawal rates.
In the three post hoc analyses, patients were stratified by (a) whether they received low-dose (4 and 6 mg/day) or high-dose (8, 10, and 12 mg/day) perampanel during the maintenance period; (b) whether they received perampanel added to first- and second-line ASM monotherapy at baseline; and (c) the specific background ASM monotherapy to which perampanel was added (limited to background ASMs taken by ≥3 patients) and the perampanel maintenance dose (4, 6, 8, 10, and 12 mg/day). Statistical analyses of differences in efficacy endpoints between the low- versus high-dose perampanel maintenance dose and first- versus second-line ASM monotherapy subgroups were conducted using Wilcoxon rank-sum test. A p-value of less than 0.05 was considered statistically significant in all tests. Safety analyses were conducted in a descriptive manner.
Results
Patients
The FAME study enrolled 106 patients with FOS, with 85 included in the FAS.
18 At baseline in the total FAS population, 57.6% of patients were female, mean age of patient age was 42.3 years, mean years since an epilepsy diagnosis was 10.9 years, and the mean FOS rate was 4.1 per 28 days.
18 Demographics and baseline characteristics of the FAS subgroups stratified by perampanel dose and the number of baseline ASM monotherapies are summarized in
Table 1. In the low-dose perampanel group (4 or 6 mg/day), the cause of epilepsy was unknown in 71.4% of patients; in the remaining patients, seven patients had head injury/cranial trauma (post-traumatic epilepsy/post-neurosurgery), one patient had stroke (post-stroke epilepsy), four patients had structural brain anomalies or malformations (tuberous sclerosis etc.), four patients had vascular brain anomalies, and seven patients had other causes (including spastic cerebral palsy, hippocampal sclerosis, and calcification on the left frontal area). In the high-dose perampanel group (8, 10, or 12 mg/day), the cause of epilepsy was unknown in 53.3% of patients; in the remaining patients, three patients had structural brain anomalies or malformations (tuberous sclerosis etc.), one patient had vascular brain anomalies, and two patients had other causes (e.g., temporal lobe epilepsy or perinatal injury).
Seizure-control outcomes
Most patients (82.4%; 70/85) received maintenance treatment with low-dose perampanel, with 61.4% (43/70) of these receiving 4 mg/day and 38.6% (27/70) receiving 6 mg/day (
Table 1). The remaining patients (17.6%; 15/85) received high-dose maintenance perampanel, with 80% (12/15) of these receiving 8 mg/day, 13.3% (2/15) receiving 10 mg/day, and 6.7% (1/15) receiving 12 mg/day (
Table 1).
During the maintenance period, the 50% responder rate was 88.6% and 40.0% in the low-dose perampanel and high-dose per-ampanel groups, respectively (
p<0.001). The corresponding 75% responder rates were 78.6% and 40.0% (
p=0.009), and seizure-freedom rates were 54.3% and 13.3% (
p=0.004) (
Fig. 1A). The median percent reduction in seizure frequency per 28 days was 100% with low-dose perampanel and 16.7% with high-dose per-ampanel (
p<0.001) (
Fig. 1B).
Almost all patients (92.9%, n=79) in the FAS received perampanel as the first add-on to their first-line ASM monotherapy, with the remaining six patients (7.1%) receiving perampanel as a first add-on to second-line ASM monotherapy (
Table 1). The first ASM monotherapy group had significantly higher 50% responder rates with add-on per-ampanel than the second ASM monotherapy group (83.5% vs. 33.3%;
p=0.013) (
Fig. 2A). In the first and second ASM mono-therapy groups, the respective 75% responder rates were 76.0% and 16.7% (
p=0.006), seizure-freedom rates were 50.6% and 0.0% (
p=0.027) (
Fig. 2A), and the median percent reductions in seizure frequency per 28 days was 100.0% and 21.8%; (
p=0.004) (
Fig. 2B).
Post hoc analysis of patients stratified by concomitant ASMs at baseline included 82 patients following the removal of three patients from the FAS who received topiramate (n=2) or zonisamide (n=1) due to very low patient numbers. Consequently, analysed patients received five different concomitant ASMs at baseline, most commonly levetiracetam (41.5%; n=34), followed by carbamazepine (24.4%; n=20), oxcarbazepine (20.7%, n=17), lamotrigine (8.5%; n=7), and valproic acid (4.9%, n=4). Most patients received maintenance doses of add-on perampanel of 4 mg/day (51.2%, 42/82), 6 mg/day (31.7%; 26/82), or 8 mg/day (13.4%; 11/82), with only a few receiving 10 mg/day (2.4%; 2/82), or 12 mg/day (1.2%; 1/82).
Fig. 3 shows seizure-control outcomes by concomitant ASM use and perampanel dose during the maintenance period. The 50% responder rate was highest when perampanel 4 mg/day was added to levetiracetam (85.0%, 17/20), carbamazepine (100.0%, 7/7), oxcar-bazepine (100.0%, 7/7), lamotrigine (100.0%; 6/6), and valproic acid (100.0%; 2/2) (
Fig. 3A). Seizure-freedom rates were highest with add-on perampanel 4 mg/day to carbamazepine (71.4%, 5/7), oxcarbazepine (85.7%, 6/7), and valproic acid (100.0%, 2/2), and add-on perampanel 6 mg/day to oxcarbazepine (71.4%, 5/7) (
Fig. 3B). The greatest median reduction in seizure frequency per 28 days was for perampanel 4 mg/day added to levetiracetam, carbamaze-pine, oxcarbazepine, or valproic acid (all 100.0%), followed by per-ampanel 4 mg/day added to lamotrigine (96.9%) (
Fig. 3C).
Safety outcomes
In
post hoc analyses, the SAS included 88 patients in the per-ampanel maintenance dose analysis, 102 in the number of ASM monotherapies analysis, and 85 in the concomitant background ASM analysis (
Table 2). At least one TEAE was reported by most (77.5%; 77/102) patients in FAME, which were generally mild (64.8%) or moderate (15.7%) in severity.
18 The proportion of patients reporting at least one TEAE was comparable between those receiving low-dose and high-dose perampanel (76.7% and 60.0%), and between those receiving first and second ASM monotherapy at baseline (75.3% and 77.8%) (
Table 2).
The most common (reported in >5 patients) TEAEs were dizziness, headache, and somnolence, all involving the central nervous system (
Table 2). Dizziness was the most common TEAE in the low- and high-dose perampanel (49.3% and 46.7%) groups, first- and second-line ASM monotherapy (51.6% and 33.3%) bgroups, and most concomitant background ASM and perampanel dose groups (
Table 2). Headache was reported in approximately 10% of patients receiving low-dose perampanel and first-line ASM monotherapy, but was not reported in the small subgroups of patients receiving high-dose perampanel or second-line ASM monotherapy (
Table 2). Somnolence was reported in approximately 10% of patients regardless of whether they were receiving low- or high-dose perampanel, or first- or second-line ASM monotherapy.
The incidence of the most common TEAEs varied to a greater extent when patients were stratified by concomitant background ASM and perampanel dose, which generated a large number of categories with few (or no) patients in each subgroup and precluded identification of any clear trends. Somnolence was reported in three of 22 patients (13.6%) receiving background carbamazepine: in the 6 mg/day (n=2) and 8 mg/day (n=1) perampanel subgroups; and three of 17 patients (17.6%) receiving background oxcarbazepine: 1 in each perampanel 4, 6, and 8 mg/day subgroups (
Table 2). Dysarthria was reported by a total of 5/102 (4.9%) patients in FAME, all of whom were receiving first ASM monotherapy, and three-quarters of whom were receiving low-dose perampanel. When stratified by background ASM, dysarthria was reported in two of the 85 patients in the SAS, both of whom were receiving perampanel 4 mg/day as an add-on to background levetiracetam.
Serious TEAEs were reported in 7.8% of patients (8/102) in FAME.
18 When stratified in
post hoc analyses, serious TEAEs occurred in 6.9% and 6.7% of patients in the low- and high-dose groups, respectively, and 6.5% and 14% of patients in the first- and second-line ASM monotherapy groups, respectively (
Table 2). The study discontinution rate due to a TEAE was 13.7% (14/102) in the FAME study.
18 In
post hoc analyses, this rate was 5.5% in the low-dose group and 0% in the high-dose group, and was 14.0% and 11.1% in the first- and second-line ASM monotherapy groups, respectively (
Table 2). Given the small patient numbers in the concomitant background ASM subgroups, there were no clear differences in the proportions of patients reporting serious TEAEs or those discontinuing the study due to a TEAE.
Discussion
The three post hoc analyses of FAME described here found that per-ampanel improves seizure control as the first add-on to first- or second-line ASM monotherapy, indicating its overall usefulness as add-on therapy early in the treatment of FOS. Low-dose (4 and 6 mg/day) maintenance perampanel was more effective in achieving seizure control than high-dose (8, 10, and 12 mg/day) perampanel. The increased efficacy of lower doses may be due to the relative responsiveness of patients with poor responders needing higher doses of perampanel, although it should be noted that many more patients received low-dose (n=70) compared with high-dose (n=15) perampanel. Based on tolerability, only 17.5% of patients were titrated to higher doses during the 12-week titration phase, and patients entered the maintenance phase receiving their last tolerated dose. Nevertheless, the high responder rates in the low-dose group suggest that many patients may achieve seizure control with relatively low perampanel doses, whereas others may require titration to higher doses if tolerated.
Perampanel may be used early in the treatment of FOS, as its use as the first add-on to both first- and second-line ASM monotherapy improved seizure control. Similar results were shown in an observational real-world setting when perampanel was the first or second add-on to ASM therapy.
16 As with other ASMs, seizure control rates decrease when perampanel is added as subsequent-line therapy in patients with treatment-resistant FOS.
9–
11,
17,
19
The perampanel maintenance dose (low vs. high), or use as an add-on to first- or second-line ASM monotherapy, did not appear to influence the tolerability profile of perampanel, with most TEAEs being mild in severity and only a few patients discontinuing the study. The overall tolerability profile of perampanel in the
post hoc analyses did not differ from that previously reported in pooled phase III trials.
8,
19 Dizziness was the most common TEAE overall in FAME (50% of patients)
18 and each of the
post hoc analysis subgroups, and was usually mild or moderate and resolved in all patients. Central nervous system-related TEAEs, such as dizziness, somnolence, and headache, have been consistently reported in clinical and real-world studies of perampanel,
6,
8,
20,
21 as well as in studies of other ASMs.
22 Of note, the incidence of dizziness may decrease if dose up-titration is slow (performed at ≥2-week intervals) rather than fast (performed at <2-week intervals), as shown by a previous
post hoc analysis of FAME.
18
The first-line ASM taken as concomitant background ASM therapy with add-on perampanel may be expected to influence the incidence of some TEAEs. For example, dysarthria was reported only in patients receiving contaminant background levetiracetam. However, the small number of patients in each background ASM and add-on per-ampanel subgroup makes it difficult to make any conclusions regarding the relative tolerability of these ASM-perampanel combinations.
The original FAME study was limited by being conducted only in South Korean patients, its open-label and non-comparative design, and its relatively small overall study population.
18 The latter limitation is further compounded in the current
post hoc analyses, which consider relatively small subgroups from the original FAME study. Direct comparisons between subgroups are limited by the much higher number of patients in the low-dose group (n=70) than in the high-dose group (n=15), and in the first-line ASM monotherapy group (n=79) than in the second monotherapy group (n=6), and the small number of patients (ranging from n=4 to n=34) receiving each of the first-line ASMs as background therapy. Nevertheless, these analyses help to further characterize the clinical profile of add-on per-ampanel in patients with FOS.
In conclusion, these post hoc analyses of FAME provide supportive data for the use of perampanel as an effective and well-tolerated first add-on treatment in patients with FOS. Results show that per-ampanel may provide seizure control regardless of the perampanel maintenance dose and number of prior ASM monotherapies.
Conflicts of Interest
Conflict of Interest
Ji Woong Lee and Min Young Kim are employees of Eisai Korea Inc. All other authors have no conflict of interest to declare.
Acknowledgements
Under the guidance of the authors, professional medical writing and editorial support was provided by Katherine A. Lyseng-Williamson BSc. Pharm and David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from Eisai Korea Inc.
Funding for the FAME study and post hoc analyses was provided by Eisai Korea Inc.
Figure 1
Seizure-control outcomes in perampanel recipients stratified by perampanel daily maintenance dose (low vs. high), full analysis set (n=85). (A) Responder rates (50%, 75%, and seizure-free) and (B) median percentage reduction from baseline seizure frequency per 28 days.

Figure 2
Seizure-control outcomes in perampanel recipients stratified by first and second anti-seizure medication (ASM) monotherapy use at baseline, full analysis set (n=85). (A) Responder rates (50%, 75%, and seizure-free) and (B) median percent reduction from baseline seizure frequency per 28 days.

Figure 3
Seizure-control outcomes in perampanel recipients stratified by baseline concomitant anti-seizure medication and daily perampanel maintenance dose, full analysis set (n=83). (A) 50% responder rates, (B) seizure-free responder rate, and (C) median percentage reduction from baseline seizure frequency per 28 days. aBased on data from 19/20 patients.
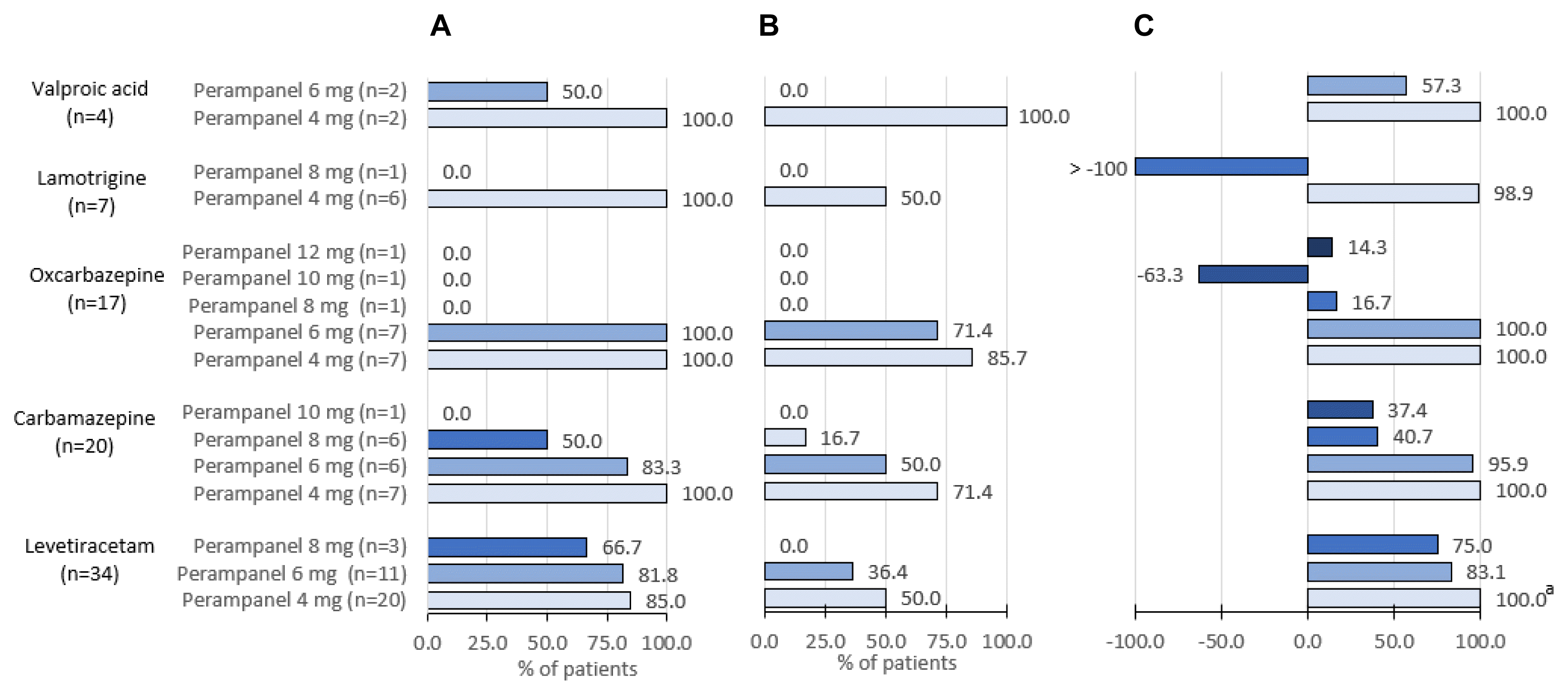
Table 1
Patient demographics and baseline characteristics in patients receiving perampanel as first add-on therapy in subgroups stratified by perampanel daily maintenance dose (low vs. high), and ASM monotherapy use at baseline (first- vs. second-line), full analysis set (n=85)
|
Parameter |
Perampanel maintenance dose |
ASM monotherapy use at baseline |
|
|
|
Low: 4–6 mg/day (n=70) |
High: 8–12 mg/day (n=15) |
First-line (n=79) |
Second-line (n=6) |
|
Age* (years) |
43.4±14.2 |
37.3±12.5 |
41.9±13.7 |
47.8±18.9 |
|
Female |
41 (58.6) |
8 (53.3) |
45 (57.0) |
4 (66.7) |
|
Age at diagnosis (years) |
32.2±15.9 |
28.0±12.4 |
31.1±14.8 |
37.0±22.5 |
|
Duration of disease† (years) |
11.1±9.9 |
9.3±6.2 |
10.8±9.4 |
10.8±9.6 |
|
Perampanel dose (mg/day) |
– |
– |
5.3±1.6 |
7.3±3.0 |
|
No. of prior/current ASM monotherapies |
|
1 |
66 (94.3) |
13 (86.7) |
– |
– |
|
2 |
4 (5.7) |
2 (13.3) |
– |
– |
|
Perampanel daily dose |
|
4 mg |
43 (61.4) |
– |
42 (53.2) |
1 (16.7) |
|
6 mg |
27 (38.6) |
– |
24 (30.4) |
3 (50.0) |
|
8 mg |
– |
12 (80.0) |
12 (15.2) |
0 (0.0) |
|
10 mg |
– |
2 (13.3) |
1 (1.3) |
1 (16.7) |
|
12 mg |
– |
1 (6.7) |
0 (0.0) |
1 (16.7) |
|
First-/second-line ASM monotherapy |
|
Levetiracetam |
– |
– |
31 (39.2) |
3 (50.0) |
|
Carbamazepine |
– |
– |
20 (25.3) |
0 (0.0) |
|
Oxcarbazepine |
– |
– |
14 (17.7) |
3 (50.0) |
|
Lamotrigine |
– |
– |
7 (8.9) |
0 (0.0) |
|
Valproic acid |
– |
– |
4 (5.1) |
0 (0.0) |
|
Topiramate |
– |
– |
2 (2.5) |
0 (0.0) |
|
Zonisamide |
– |
– |
1 (1.3) |
0 (0.0) |
Table 2
TEAEs reported in perampanel recipients stratified by daily perampanel maintenance dose, and ASM monotherapy use at baseline, and concomitant background ASM use and daily perampanel maintenance dose, safety analysis set (n=102)
|
Subgroup |
Type of TEAE |
|
|
Any |
Most common (reported by >5 pts) |
Serious |
Leading to discontinuation |
|
|
Dizziness |
Headache |
Somnolence |
|
Stratified by perampanel maintenance dose (n=88) |
|
|
|
|
|
|
|
Low: 4–6 mg/day (n=73) |
56 (76.7) |
36 (49.3) |
7 (9.6) |
8 (11.0) |
5 (6.9) |
4 (5.5) |
|
High: 8–12 mg/day (n=15) |
9 (60.0) |
7 (46.7) |
0 (0.0) |
2 (13.3) |
1 (6.7) |
0 (0.0) |
|
Stratified by ASM monotherapy at baseline (n=102) |
|
|
|
|
|
|
|
First-line (n=93) |
70 (75.3) |
48 (51.6) |
9 (9.7) |
9 (9.7) |
6 (6.5) |
2 (22.2) |
|
Second-line (n=9) |
7 (77.8) |
3 (33.3) |
0 (0.0) |
1 (11.1) |
13 (14.0) |
13 (14.0) |
|
Stratified by concomitant ASM and perampanel dose (n=85) |
|
|
|
|
|
|
|
Levetiracetam (n=35) |
|
|
|
|
|
|
|
4 mg/day (n=21) |
17 (81.0) |
12 (57.1) |
2 (9.5) |
1 (4.8) |
2 (9.5) |
2 (9.5) |
|
6 mg/day (n=11) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
8 mg/day (n=3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Carbamazepine (n=22) |
|
|
|
|
|
|
|
4 mg/day (n=8) |
3 (37.5) |
2 (25.0) |
1 (12.5) |
0 (0.0) |
0 (0.0) |
1 (12.5) |
|
6 mg/day (n=7) |
4 (57.1) |
2 (28.6) |
0 (0.0) |
2 (28.6) |
0 (0.0) |
0 (0.0) |
|
8 mg/day (n=6) |
2 (33.3) |
2 (33.3) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
0 (0.0) |
|
10 mg/day (n=1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Oxcarbazepine (n=17) |
|
|
|
|
|
|
|
4 mg/day (n=7) |
6 (85.7) |
4 (57.1) |
0 (0.0) |
1 (14.3) |
0 (0.0) |
0 (0.0) |
|
6 mg/day (n=7) |
5 (71.4) |
2 (28.6) |
1 (14.3) |
1 (14.3) |
0 (0.0) |
0 (0.0) |
|
8 mg/day (n=1) |
1 (100.0) |
1 (100.0) |
0 (0.0.0) |
1 (100.0) |
0 (0.0) |
0 (0.0) |
|
10 mg/day (n=1) |
1 (100.0) |
1 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
12 mg/day (n=1) |
1 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (100.0) |
0 (0.0) |
|
Lamotrigine (n=7) |
|
|
|
|
|
|
|
4 mg/day (n=6) |
6 (100.0) |
5 (83.3) |
1 (16.7) |
0 (0.0) |
1 (16.7) |
1 (16.7) |
|
8 mg/day (n=1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Valproic acid (n=4) |
|
|
|
|
|
|
|
4 mg/day (n=2) |
2 (100.0) |
1 (50.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
6 mg/day (n=2) |
2 (100.0) |
2 (100.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
References
1. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80.


3. National Institute for Health and Care Excellence (NICE). Epilepsies: diagnosis and management [Internet]. London: NICE; 2012. [cited 2021 May 12]. Available at : https://www.nice.org.uk/guidance/CG137
.
4. Byun JI, Kim DW, Kim KT, et al. Treatment of epilepsy in adults: expert opinion in South Korea. Epilepsy Behav. 2020;105:106942


5. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–30.


6. US Food and Drug Administration (FDA). Fycompa (perampanel) tablets and oral suspension: prescribing information [Internet]. Silver Spring (MD): FDA; 2021. [cited 2021 May 12]. Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202834s011lbl.pdf
.
7. Eisai Korea, Inc. Fycompa (perampanel) tablets and oral suspension [Internet]. Cheongju: Ministry of Food and Drug Safety; 2015. [cited 2021 May 12]. Available at : https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=201504769
.
8. Rugg-Gunn F. Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia. 2014;55:Suppl 1. 13–5.


10. French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive per-ampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–25.


11. Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–15.


14. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9.


15. Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9:464–8.


16. Abril Jaramillo J, Estévez María JC, Girón Úbeda JM, et al. Effectiveness and safety of perampanel as early add-on treatment in patients with epilepsy and focal seizures in the routine clinical practice: Spain prospective study (PERADON). Epilepsy Behav. 2020;104:Pt A. 106917


17. Villanueva V, Garcés M, López-González FJ, et al. Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: the FYDATA study. Epilepsy Res. 2016;126:201–10.


18. Kim JH, Kim DW, Lee SK, et al. First add-on perampanel for focal-onset seizures: an open-label, prospective study. Acta Neurol Scand. 2020;141:132–140.


19. Steinhoff BJ, Ben-Menachem E, Ryvlin P, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–9.


21. Hwang SK, Lee YJ, Nam SO, et al. Real-life effectiveness and tolerability of perampanel in pediatric patients aged 4 years or older with epilepsy: a Korean national multicenter study. J Clin Neurol. 2020;16:53–9.






