Introduction
Headache is often associated with epilepsy. Headache is a common feature of several childhood epilepsy syndromes and can also occur in adult patients with epilepsy as pre-ictal, ictal, or post-ictal phenomenon.1 In addition, headache can be a sole manifestation of epileptic seizure, known as ictal epileptic headache. However, this condition is considered as exceptionally rare with difficulty in demonstrating ictal epileptiform discharges and response to intravenous antiseizure medication administration during a short-lasting headache.2
Aura in epilepsy is generally considered as a phenomenon in patients with focal seizures with localizing value. However, several studies have shown that patients with generalized seizures also experienced various types of auras including headache.3,4 In patients with ictal epileptic headache, recurrent generalized spike-wave discharges are one of the most common electroencephalogram (EEG) findings, suggesting a close relationship between headache and generalized seizures.5 However, studies on the frequency and characteristics of headache as an aura in patients with generalized seizures are insufficient. The objective of this study was to determine the frequency and characteristics of headache as an aura in patients with generalized seizures.
Methods
We retrospectively reviewed the epilepsy registry of consecutive epilepsy patients presenting at the Epilepsy Clinic in our hospital between March 2008 and February 2022 with confirmation of diagnosis of generalized seizures, adequate evaluation with epilepsy protocol magnetic resonance imaging (MRI) and EEG, and available follow-up for at least 6 months. Patients with no epileptiform discharge at the initial study underwent repeated EEG studies up to three times to increase the accuracy of diagnosis. Classification of epileptic seizures was based on the clinical semiology and the results of MRI and EEG, but the presence of generalized epileptiform discharge was considered essential for the diagnosis of generalized seizure. We carefully examined the EEGs showing generalized epileptiform discharges to exclude the possibility of focal seizure onset with the characteristics of secondary bilateral synchrony, including consistent ‘lead-in’ discharges from one hemisphere and presence of focal interictal discharges confined to one hemisphere. This study protocol was approved by our institutional review board. Informed consent was waived because of the retrospective nature of this study.
Aura was defined as a subjective warning symptom prior to clinical seizures regardless of the presence of impairment of consciousness. The presence of aura including headache was initially evaluated during history taking with open questioning, and the detailed information of aura was assessed using a semi-structured questionnaire.6
Clinical features included gender, age at seizure onset, types of epileptic seizures, the presence of aura, and types of auras. We compared clinical characteristics between the patients with and without headache as an aura using Student’s t-test for continuous variables and chi-squared test or Fisher’s exact test for categorical variables.
Results
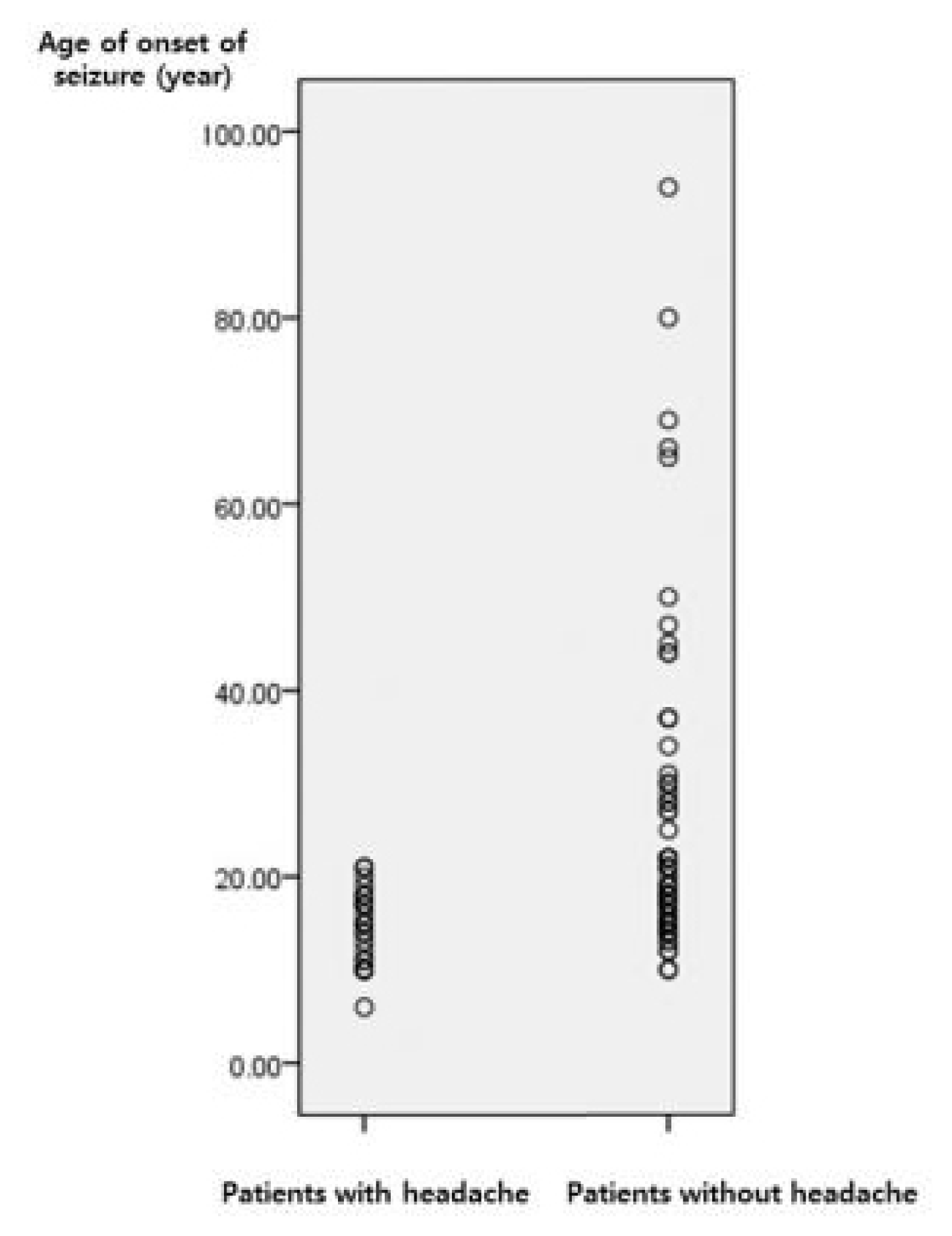
In our epilepsy registry, 1,632 patients were classified as having focal seizures, while 162 patients were classified as having generalized seizures. Sixty patients were excluded from the analysis due to insufficient follow-up or lack of detailed clinical information and 102 patients were included in the final analysis. The patients were 44 males and 58 females. The mean age of onset of seizures was 22.2±14.8 (range: 6–94 years). The most frequent seizure types were absence seizures in eight patients, myoclonic seizures in 54 patients, and generalized tonic-clonic seizures in 40 patients. Aura was documented in 45 patients (45/102, 44.1%) and 11 patients (11/102, 10.5%) experienced at least 2 types of auras. Headache was the most common aura reported in 26 patients (26/102, 25.5%), followed by dizziness (17 patients), palpitation (four patients), cephalic (four patients), nausea (three patients), and visual symptoms (two patients). There were no differences in gender and seizure type between patients with and without headache as an aura. However, the age of onset of seizures was significantly lower in patients with headache as an aura than in patients without headache (14.8±3.8 vs. 24.7±16.2, p=0.003, Table 1). All patients with headache as an aura had onset of seizures before their early twenties, whereas in patients without headache, the age of onset of seizures was widely distributed (Fig. 1). There was no difference in the age of onset of seizures between patients with and without aura other than headache (21.6±15.1 vs. 22.5±14.7, p=0.78).
Discussion
Aura is considered as the subjective experience of focal cortical excitation. The presence of auras and other lateralizing clinical features are traditionally regarded as an indication that seizures are focal rather than generalized in onset. However, previous studies have shown that the proportion of patients with aura does not differ between patients with focal and generalized seizures.3 Diverse auras have been reported in patients with generalized seizures and head-ache being one of the most common auras in these patients.3,4 In our study, more than 40.0% of patients with generalized seizures experienced auras, and headache was reported as the most common aura in more than a quarter of the patients (26/102, 25.5%). Although proportions of patients with auras and those with headache as an aura were higher than expected in our study, the proportion of patients with auras was even higher (up to 70.0%) in previous studies with closed-ended questions.3,4 Since the proportion of headache as an aura has been reported to be only 1.0–2.0% of patients with focal seizures,7,8 our study suggests that headache is a more common aura in patients with generalized seizures than in patients with focal seizures.
Our study showed that there was a significant difference in the age of onset of seizures between patients with and without headache as an aura. Age of onset of seizures was widely distributed among patients without headache, whereas patients with headache had a younger age of seizure onset before their early twenties. No satisfactory explanation was found for this result. However, previous studies on patients with focal seizures have also shown the difference in aura and ictal semiology according to age of onset. Difference in the maturation stage of the brain at the time of onset of illness has been proposed as an explanation for this interesting phenomenon.9 Therefore, it can be hypothesized that the chance of headache as an aura in patients with generalized seizures depends largely on the stage of the brain maturation rather than on type of seizure and other clinical features of the seizures. Actually, headache was a common feature of childhood epilepsy syndromes of unknown etiology including benign epilepsy with centrotemporal spikes, idiopathic childhood epilepsy of Gastaut type, and even childhood absence epilepsy,10,11 and early reports on the association between epilepsy and headache were based on clinical observations of pediatric patients who experienced recurrent episodes of ‘intermediate’ in type between migraine and epilepsy.12
Another interesting finding of our study was that many patients had auras that are common features of migraine, such as dizziness, nausea, and visual symptoms. Due to the high comorbidity of migraine and epilepsy in childhood, some patients in our study might confuse aura in migraine with aura in epilepsy. However, we believe that patients with generalized seizures may actually experience symptoms of migraine before seizures because excessive cortical excitation is a possible common pathophysiology of these two strongly related diseases, migraine and epilepsy.
This study had several limitations. First, the study included a small number of patients over a limited period in a tertiary referral epilepsy clinic; therefore, results cannot be generalized to all patients with seizures. Second, more than one-third of patients with generalized seizures were excluded from analysis due to insufficient clinical information or short-term follow-up, which can be a cause of selection bias. Finally, because the presence and type of aura were assessed only with the open questions at the time of history taking, the proportion of patients with aura might have been underestimated. However, our study has clinical significance in that it shows a high incidence of headache as an aura in patients with generalized seizures and its relationship with the age of onset of seizures. Finally, because we assessed the presence of aura including headache during history taking and its time relationship with clinical seizure was not confirmed via EEG correlation, it is possible that a portion of our patients might confuse prodrome symptoms in epilepsy with aura. Further studies with are needed to clearly demonstrate the accurate incidence of headache as an aura in patients with generalized seizures and the effect of age of onset of seizures on the development of headache.