Introduction
“Ketogenic diet” means a diet that generates ketone bodies, including acetoacetate (ACA), β-hydroxybutyrate (BHB), and acetone as the name indicates. Ketogenic diet is consisted of high fat, low carbohydrate and adequate protein. In spite of the several difficulties in managing the diet, the ketogenic diet is now widely used and recognized as definitely effective treatment for intractable epilepsies.1,2
Despite the scanty knowledge about the mechanisms of action of the diet, we have noticed that its success depends on the ketosis of the patients.3 It has also been reported that higher plasma BHB levels are correlated with anticonvulsant effects in mice fed a ketogenic diet4 and that ACA and acetone have been found to have an anticonvulsant action in mice.5 These data are consistent with the early hypothesis that the anticonvulsant efficacy of the ketogenic diet involves the direct actions of the ketone bodies.6 Recently, we reported that BHB, one of the ketone bodies, might be directly anticonvulsant in other seizure model7,8 and in young animal with pilocarpine-induced seizures.9
On the other hand, the ketogenic diet is so far known to be more effective in younger individuals,10 and this age-dependent efficacy is considered due to the different metabolic control.11 Our present study was designed to investigate whether exogenous injection of BHB has a direct anticonvulsant effect on pilocarpine-induced seizures in mature mice as well.
Methods
Eighty-two male ICR mice (Folas International, Korea) at postnatal day 49 were used for all the experiments. Animals were housed in a room maintained at 22±3°C with an alternating 12-h light/dark cycle, and fed normal diet. Experiments have been approved by the Institutional Animal Care and Use Committee of the Inje University and conform to the Revised Guide for the Care and Use of Laboratory Animals [NIH GUIDE, 25(28), 1996].
All mice were pretreated by intraperitoneal injection of 1 mg/kg scopolamine methylbromide (Sigma, USA) 30 min prior to pilocarpine administration. Experimental mice (n=42) were injected intraperitoneally with 20 mmol/kg (R)-3-hydroxybutyric acid sodium salt (BHB; Fluka, USA), 15 min prior to pilocarpine administration; control animals (n=40) were administered the same volume of normal saline. Blood samples of 24 mice were collected via the tail vein immediately before pilocarpine administration in order to measure blood BHB levels using a Keto-Site test kit (GDS Diagnostics, USA). Seizures were chemically induced in both BHB-treated and control mice by intraperitoneal injection of a single 300 mg/kg dose of pilocarpine (Sigma, USA).
After induction of seizure, all mice were monitored for seizure behaviors for 2 h. Seizure behaviors were categorized into five different grades according to the previously defined Racine scale.12 Twelve mice from each group were graded according to the maximum seizure behavioral response observed.
Differences between two groups were statistically analyzed using unpaired Student’s t-tests; a value of p<0.05 was considered significant. All data are presented as means (±SD).
Results
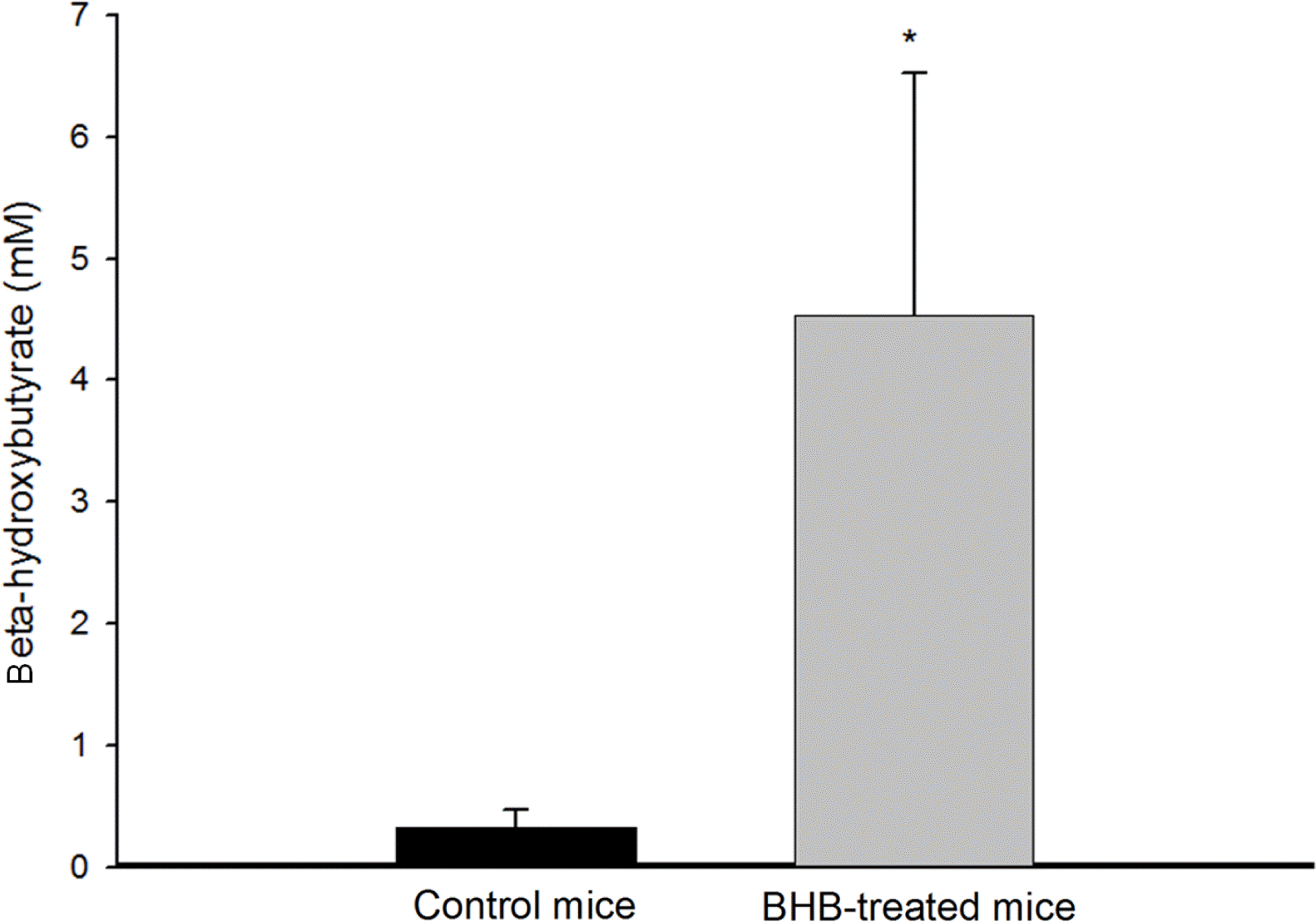
After intraperitoneal administration of BHB, we confirmed that blood BHB levels in treated mice were significantly increased compared with those in controls (4.54±1.99 mM vs. 0.32±0.15 mM, p < 0.001) (Figure 1).
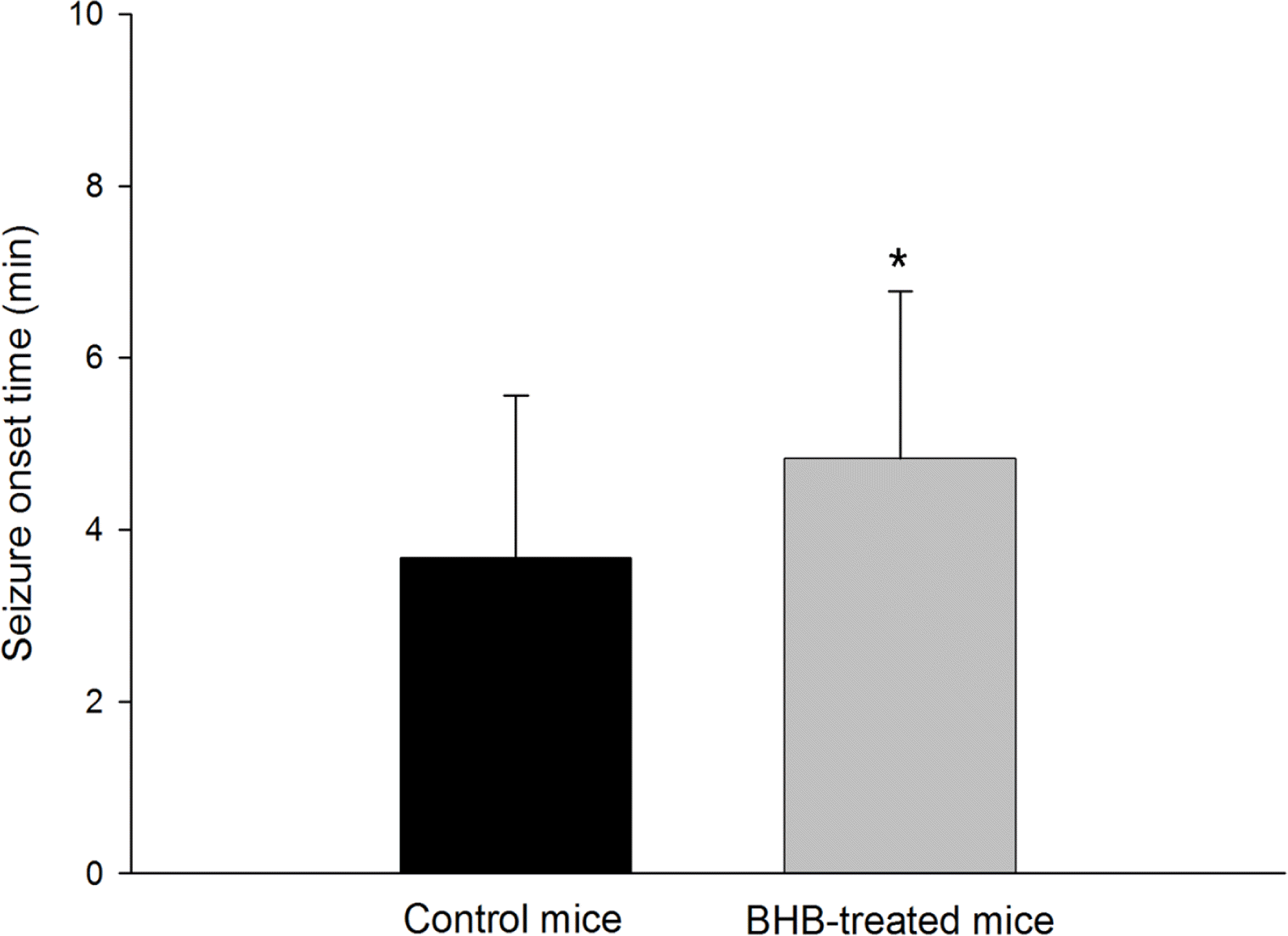
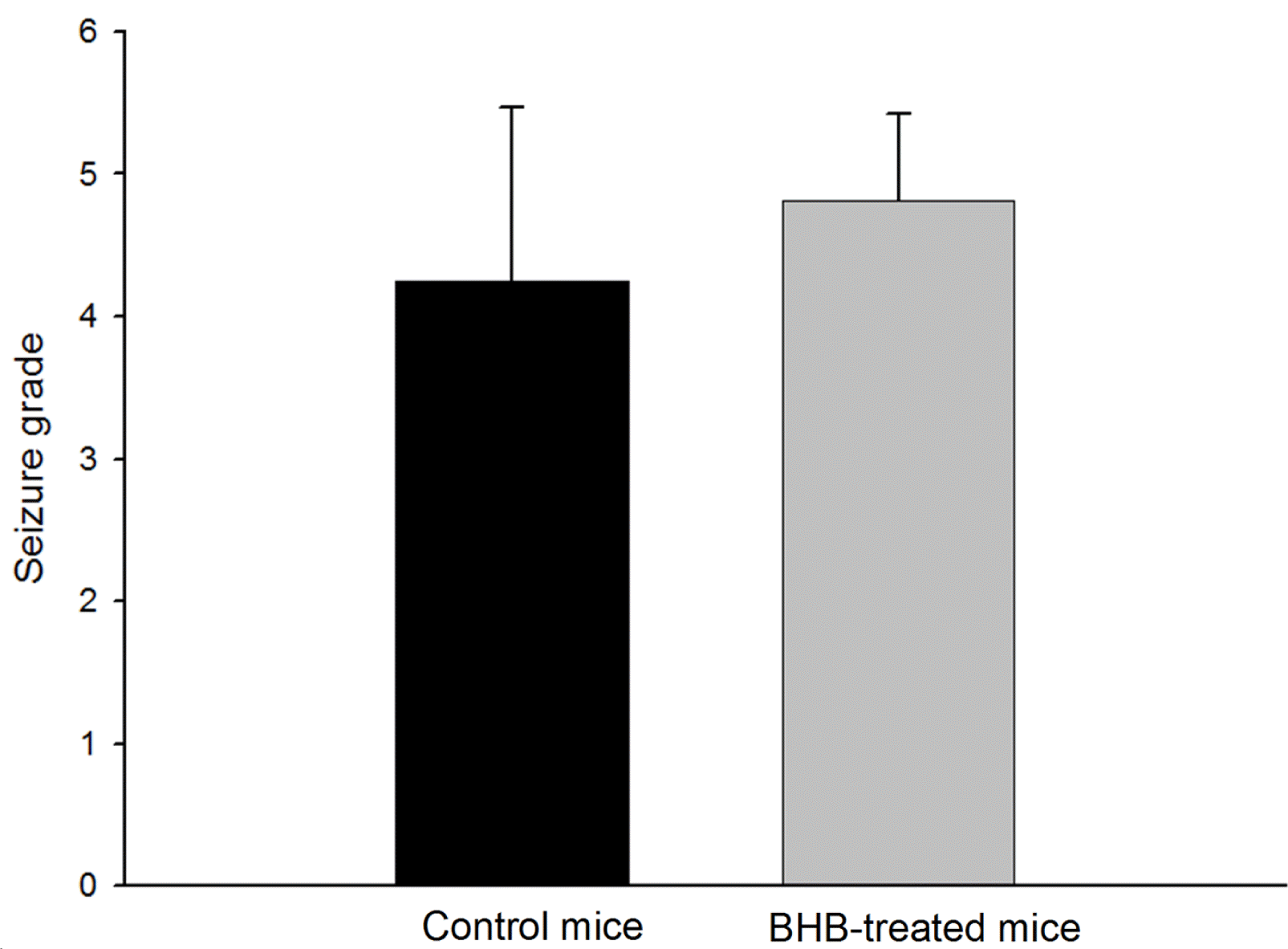
To ascertain the effect of the BHB treatment, the latency to the onset of seizures and seizure behaviors were monitored. The mean (±SD) latency to the onset of seizure after pilocarpine injection was significantly prolonged in BHB-treated mice compared with controls (4.83±1.95 min vs. 3.67±1.90 min, p<0.01) (Figure 2). During the 2 hour monitoring of seizure behaviors to determine seizure severity, all monitored mice developed typical seizure behaviors after pilocarpine administration. However, the seizure behavior grades, the seizure severity, were not significantly different between BHB- treated and control mice (4.82±0.60 vs. 4.25±1.22, Figure 3).
Discussion
There is no more controversy that the ketogenic diet is effective against refractory epilepsy. It has also been shown to be effective at increasing thresholds to flurothyl-induced seizures in several inbred strains of mice.10,13,14 The mechanism of action of the diet, however, has not been answered yet1 although several mechanisms have been hypothesized. Proposed mechanisms include direct modulation of ion channels via ketone bodies,15 activation of ATP-sensitive potassium (KATP) channels via glucose restriction,16 increased noradrenergic tone,14 and increased GABAergic inhibitory signaling.17
Here, the main hypothesis of our present study is that ketone body, a product of ketogenic diet, has direct anticonvulsant effects. We have already examined this hypothesis in previously published studies.7–9 We chose the most stable physiologic ketone body-BHB-as the representative ketone body for the present study. In our study, injection of BHB could mimic the conditions under fasting and increased blood BHB level around 4–5 mM as previously shown. And this increased BHB was accompanied by an increase in the threshold for pilocarpine-induced seizures in these mature animals. Although seizure severity, measured as seizure behavior grade, was not significantly different, the latency to the onset of seizure induced by pilocarpine was significantly prolonged in BHB-treated mice compared with controls. These results are consistent with our previous reports that treatment with BHB raises the threshold in young animals with flurothyl-induced seizures7,8 and young mice with pilocarpine-induced seizures.9 This shows that BHB has a direct anticonvulsant effect and can be a main anticonvulsant component of the ketogenic diet.
The better efficacy of ketogenic diet in a younger individual10,18,19 has been observed. In animal, age-related difference of the ketogenic diet in fluorothyl seizure susceptibility was shown before.10 For this different efficacy with age, some developmental factors such as different metabolic control of the young and mature brain were suggested. Although it has been known that mature brain does not usually burn ketone bodies for energy production unless blood glucose levels are reduced,11 additional ketone body effectively reduced the seizure threshold without lowering blood glucose level in our present study. In other words, direct supplementation of BHB effectively elevates the seizure threshold in vivo without restriction of glucose in adult age group.
The present study tested the very acute effect of the ketone body, which has no association with the long-term metabolic and genetic modification produced by the ketogenic diet. Thus, the anticonvulsant action of BHB could be an acute modulation of brain activity. For example, ketone bodies can stimulate glutamic acid decarboxylase activity, which can potentially elevate GABA content in synaptosomes,20 and ketone body-induced alterations in TCA cycle metabolites (oxaloacetate, citrate, succinyl-CoA, and α-ketoglutarate) favor the formation of glutamate over aspartate. Accordingly, an attenuation of excitatory neurotransmitters (glutamate and aspartate) together with an elevation of GABA could decrease neuronal excitation and increase inhibition.20,21 Although we have little knowledge about the mechanisms of the ketogenic diet, the ketone bodies may have acute anticonvulsant actions possibly involving multiple changes in the content and distribution of brain neurotransmitters.
While the anticonvulsant effect of ketogenic diet may depend on multiple mechanisms working synergistically, the results from this study suggest that the ketone body, BHB, has a direct anticonvulsant activity in mature mice. This finding is consistent with previous studies dealing with anticonvulsant effects of the ketone bodies.3,7–9