Introduction
Epilepsy is a neuropsychiatric disorder with high prevalence among children and young adults. In India, about 10 million people suffer from epilepsy with a prevalence of about 1.9% in rural areas and 0.6% in urban locales. The greater prevalence of epilepsy in rural areas is a testament to impact of stigma that surrounds this illness on levels of treatment that Indians receive. About 95% of people in India who suffer from epilepsy are never treated for it and almost half of sufferers do not have access to anti epileptic drugs.1,2 It is the most expensive chronic neurological brain disorder in Europe.3,4 According to the World Health Organization and the World Bank, the costs of epilepsy constitute 0.5% of all diseases.5
In ancient as well as present times, epilepsy has been associated with religiosity6 and spirituality. People with epilepsy of comparable severity may differ widely in quality of life (QOL). A study considered the possible role of spirituality and it has been reported that spirituality could contribute to QOL in epilepsy.7 In another study, the complementary and alternative approaches have been successfully dem onstrated in epilepsy management.8
In ancient times this disease was considered as a sacred disease and a number of superstitious measures used to be taken to prevent/cure it. Yajurveda advocates performing of Hawan every day, morning and evening to attain spiritual enlightenment, mental peace, purification of the mind and environment.9 From time immemorial, human beings have used smoke of medicinal plants for curing disorders. Smoke produced from natural substances has been used extensively in many cultures and famous ancient physicians have described and recommended such use. Under the Saraswati-Indus civilization 7500 BC,10 the great Rishis (saints) used to perform agnihotra-yagnas to purify the environment as described in Rigveda-the most ancient compilation of knowledge on earth by sublimating the Hawan samagri (mixture of wood with odoriferous and medicinal herbs) in the fire accompanied by the chanting of Vedic mantras described in Rigveda.11 Smoke produced at high temperatures is considered as a simple way of administering a drug, which exhibits rapid pharmacological activity when inhaled. The sublimated vital elements and herbal medicines inhaled in Yagya first reach the brain, followed by lungs and other subtle components of the body. Thus, it has a direct healing effect on brain born diseases and complexities. Ayurveda also recommended nasal route as a preferred mode of administration of drugs for epilepsy.12 In addition to it, ancient scholars also recommended the patients to hold Hawans in a frequently managed manner but the people used to follow just because of superstitions. So the present manuscript is designed to highlight the scientific evidences that support possible prevention/cure of epilepsy through Hawan. The present manuscript is intended to highlight and integrate the modern and ancient concepts for treatment and prevention of epilepsy.
Biochemical/molecular view of epilepsy
Epileptic seizures caused by imbalance between excitatory and inhibitory processes in the brain are due to abnormalities in the membrane properties of neurons, changes in the ionic micro environment surrounding the neuron, decreased inhibition of neurotransmission (by gamma-amino butyric acid, GABA) or enhanced excitatory neurotransmission by the acidic amino acid glutamate. All ionotropic glutamate receptors are permeable to Na+ and K+ and it is the influx of Na+ and outflow of K+ through these channels that contribute to membrane depolarization and generation of the action potential. The n-methyl d-aspartate (NMDA) receptors also has a Ca++ channel that is blocked by Mg++ ions in the resting state, but under conditions of local membrane depolarization, Mg++ is displaced and channel becomes permeable to Ca++ ions. Influx of Ca++ tends to further depolarize the cell, and is also thought to contribute to Ca++ mediated neuronal injury under conditions of excessive neuronal activation (e.g. status epilepticus) potentially leading to cell death, a process termed excitotoxicity.13,14
In epilepsy, when the number of free radicals in the brain neurons increases, this interferes with respiratory chain in the mitochondria, destabilizes the lysosomal membranes, and lowers the convulsion threshold.15–17 Neuronal firing may lead to a number of neurochemical changes and cascades of events at the cellular and molecular level like mitochondrial dysfunction, increased ROS and nitric oxide (NO) which precedes neuronal degeneration and death with possible subsequent epileptogenesis. Experimental data indicate involvement of NO in pathophysiology of epileptic seizures by decreasing synaptosomal GABA up-take and reduced availability of GABA at the synapses leading to an increase of neuronal firing. Mitochondria are emerging as key participants in cell death because their association with an over-growing list of apoptosis-related problems.18 Peroxidation of neuronal membranes modifies their electrophysiological properties and leads to abnormal bioelectric discharges of neurons. Among diseases involving dysfunction in the mitochondrial structures, epilepsy is prominent. Mitochondria have important vital functions such as energy production, cellular harm control, neurotransmitter synthesis and free radical production however, it is still not clear which of these functions is affected in epileptic seizures.19 It is interesting to note that oxygen stress and mitochondrial dysfunction may both cause and be caused by epileptic attacks.17,20 Now a day work is focused on the possible interaction between oxidative stress resulting in disturbance of physiological signaling roles of calcium and free radicals in neurons, mitochondrial dysfunction, cell damage and epilepsy.21 Role of oxygen stress has been well demonstrated and discussed in experimental animal model of epileptic seizures.19, 22, 23
Mechanism of action of present drug module for epilepsy
The objective of the therapeutic management of seizures with medication is to control the seizures with minimal adverse side effects. Although the actions of each AED have unique characteristics and some drugs may act by multiple mechanisms, the anti-seizure actions of these drugs can be grouped into four broad categories like, modulation of voltage-dependent sodium, calcium or potassium channels; increase in GABA-ergic inhibition via actions on GABAA receptors or on GABA synthesis, reuptake, or degradation, decreased synaptic excitation via actions on ionotropic glutamate receptors; modulation of neurotransmitter release via presynaptic mechanisms. The drugs presently available for epilepsy are having renowned side effects like tolerance, dependence, and long term defects like psychosis, osteoporosis etc.
What is Hawan
Hawan is a Sanskrit word which refers to any ritual that involves making offerings into a consecrated fire. It was done by ‘Rishis ’ in early period and is an important religious practice in Hinduism where they are part of most Sanskar ceremonies. They are also prevalent in current-day Buddhism and Jainism. A consecrated fire is the central element of every Hawan ritual however the procedure and items offered to the fire vary by occasions/ceremony or by the benefit expected from the ritual. A Hawan (homam, yagya or agnihotra) is a scientific experiment in which special herbal/plant medicinal preparations (Hawan Samagri) are offered in the fire of medicinal woods ignited in a specially designed inverted pyramid shaped fire pit or container (called agni-kuñda). The specific shape and size of the agni-kuñda, the arrangement of wood pieces in it, the time-frequency and amount of Hawan Samagri account for controlled chemical processing in the fire and lead to sublimation, chemical conversion and/or transformation into vapor phase of the herbal/plant medicinal preparation leading to release of medicinal phytochemicals.24–27 The decomposition and transformation (into vapor or gaseous phase/colloidal forms, etc) of specific substances in the yagya-fire is a scientific method of subtlization of matter into energy and expanding its potential and positive effects. The electromagnetic waves generated thereby compounded with the sonic signals encoded in the mantras help in intensifying and transmitting the desired benefits of yagya in the surroundings atmosphere and far beyond.24,26
Hypothesis behind action of Hawan on epilepsy
A recent study in South Africa has been conducted on traditional healers. These healers believed that epilepsy could be caused by amafufunyana (evil spirits) and that biomedical doctors could not treat the supernatural causes of epilepsy. However, the healers believed that western medicines as well as traditional medicines could be effective in treating the epileptic seizures.28 In a recent study, it was found that majority of subjects had belief that prayer or pooja or Hawan can reduce the bad effects and ghost can be removed by tantriks/ojha for mental disorders. Although literacy level has improved in India, yet there are many false beliefs about Epilepsy.29 The hypothesis behind action of Hawan on epilepsy is integration of modern and ancient concepts. As per modern science, it is a known fact that nasal drug delivery systems are the best drug delivery systems for the diseases related to brain.30
It is to be noted here that the traditional systems of treatment of physical diseases employ medicines that are mostly administered orally and therefore, produce effects only after they have been digested and absorbed into the system. Most part of the medicine taken orally is not even utilized and absorbed by the digestive system. Such medicines may upset digestion and can have serious side effects. The same is more or less true when medicines are directly injected into the blood through intravenous route. They produce quicker results, but their adverse side effects are often more pronounced.
According to oldest ancient texts on medicine, Nasya hi shirsho dwaram means nose is the best route for administration of the drugs for the diseases related to brain and head. There are some disorders that may require a constant concentration of medicine for curative and prophylactic measure. For medication and direct delivery of drugs to the brain, drugs need to pass blood brain barrier. Other prerequisite for brain delivery is the nano-form or vapor form of drug that can be easily taken up through mucous membrane. These days the formulations are being designed in such a way that drug delivery is targeted and desired concentration of the drug is delivered at a target site where required drug concentration is needed.
The rapid intranasal delivery of therapeutic agents such as nerve growth factor to mouse brain allowed the by-passing of the blood brain barrier. The olfactory neural pathway provides both intra-neuronal (via axonal transport a highly time consuming process) and extra-neuronal (via bulk flow transport through peri-neural channels taking only few minutes) access to the brain.31 Traditional aroma-therapeutic practices, dating back thousands of years, are thus verified by 21st century neuroscience. Equally fascinating is the evidence that an odor-enriched environment increases neurogenesis in adult mouse brain.32 Since agents promoting neurogenesis in adult human brain, including the hippocampus, are being investigated in a variety of psychiatric disorders (e.g. depression, dementia and schizophrenia).33 Thus aromatherapy may have long-term protective potential in terms of regeneration is fascinating.34 One novel method of herbal delivery, called ‘Nasya’, involves intranasal delivery of dry herbal powders or medicated oils and is a practical, non-invasive, rapid, and simple method to deliver the therapeutic agents into the central nervous system (CNS).35 There are number of advantages of intranasal delivery as it bypasses the BBB, targets the CNS, reduces systemic exposure and systemic side effects. Rich vasculature and highly permeable structure of the nasal mucosa greatly enhance drug absorption, minimizes the degradation problem of peptide drugs, significantly increases accessibility to blood capillaries, and avoids destruction in the gastrointestinal tract, hepatic “first pass” elimination and gut wall metabolism allowing increased bioavailability.
The process of yagya magnifies the advantages of the desirable medicinal phytochemicals and other healthy nutritional substances. Medicines and herbs are vaporized by offering them into the sacrificial fire and enter the human body in a gaseous form through the nose, lungs and the pores of the skin. This might prove to be easiest, least taxing, least risky and most effective method of administering a medicine so as to reach every single cell of the body. Further, the thermal and associated aerodynamic characteristics of the base-fire cause the sublimated/vaporized substances to traverse and diffuse everywhere in the surroundings where yagya is being conducted.
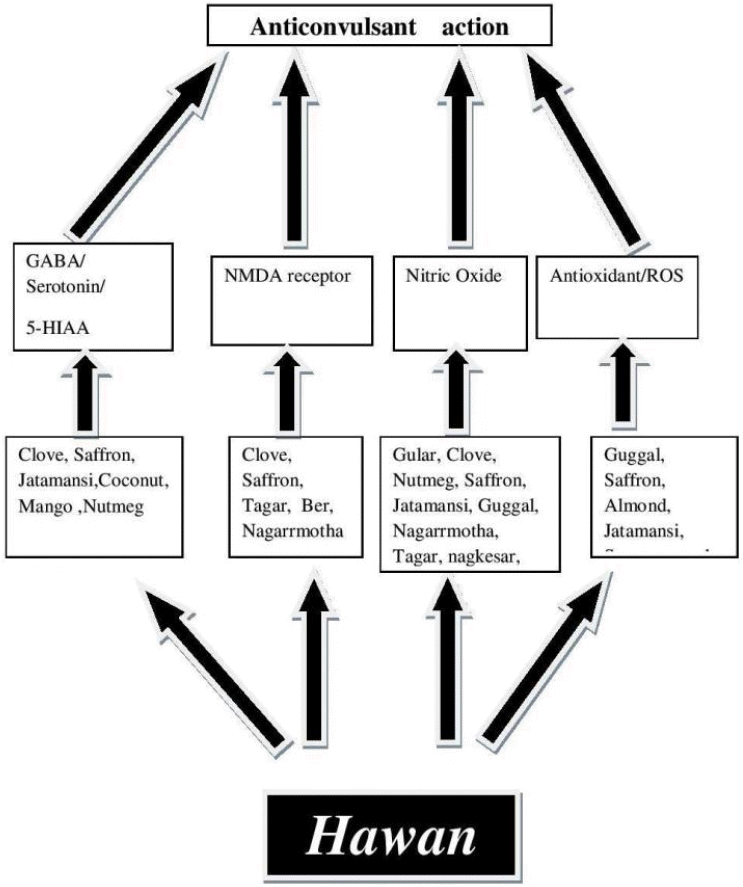
Hawan seems to be designed by the ancient scholars to fight with the diseases of the brain. The components of Hawan are having a number of volatile oils that volatilize due to high temperature of fire. The vapors of these oils enter into the central nervous system through nasal route. The routine of performing Hawan might keep the threshold value of the therapeutic components in the body and help in preventing epilepsy (Fig. 1).The scientific studies conducted on various components of Hawan clearly demonstrates that Hawan was designed for multifaceted action to clean the environment as well as to cleanse the body of the toxins responsible for causing diseases related to brain.35 Hawan fumes are not only used for the disinfection of air but also it can be environmentally oppressed for the physical, mental, intellectual and spiritual development based on nanotechnology of Hawan.27
Scientific evidences for effect of Hawan on epilepsy
The purpose of Hawan is to enhance the energy of the human body and make it healthy and progressive. The therapeutic value of Hawan is based on the ingredients used (Table 1). One of the main ingredients used is cow “Ghee” or “Clarified Butter” which has enormous beneficial properties. This ghee when burnt like oil will produce natural fumes that heal the respiratory system and clear any blood clots and bacterium affecting the nasal, lungs and veins. In the bible, the Book of Samuels, Chapter 2, “the burning of sins, using the sticks and clarified butter” infers that ghee was frequently used for fire rituals in biblical times. Essential oil constituents that penetrate the nasal passages, skin or lungs have direct actions on the autonomic nervous system that can be grouped as relaxing or stimulating in terms of basic responses such as heart rate, blood pressure and respiration, in addition to localized dermal and bronchial effects.36–42 The direct neuro-pharmacological properties of an essential oil, aroma of the oil may exert a pleasant response via the olfactory system in turn, altering the hypothalamic control of hormones and neurotransmitters. The medium chain fatty acids in pure Ghee get converted into ketones and supply the epilepsy patient brain with the energy it needs to survive and if given on a continual basis will support processes in the brain that are involved in healing and repair.
Another important ingredient in Hawan is “Camphor” from the plant Cinnamomum camphora. When the camphor is burnt in the fire ritual, the body’s breathing system is cleared quickly and the person will experience a “high” or elevated feeling during the ceremony.43
The use of CO2 as a cerebral stimulant to assist the patients suffering from lack of ventilation is common in medical world. Its use to control and cure many mental disorders is also known to medical science. Small amounts of CO2 inhaled by the persons performing Yagna acts as a stimulant and more and more aromatic fumes are inhaled which help in curing mental disorders.44
Crocus sativus L. contains important constituents like crocetin, picrocrocin, safranal that are main component for characteristic aroma. Safranal is the aglycon of picrocrocin and are responsible for many pharmacological actions.45 Saffron increased the seizure threshold, the ability of saffron in to elevate seizure threshold and block pentylenetetrazole induced (PTZ) convulsions can be attributed to its modulatory effect on GABA neurotransmission. The probable mechanism of anti epileptic activity has been shown to be by increasing the GABAergic neurotransmission. They showed that acute administration of saffron showed protection against PTZ induced convulsions. The animals showed only mild clonic convulsions followed by recovery. This may be because of their interaction with GABA benzodiazepine receptor complex. Another study also showed that pretreatment with saffron offered a significant protection both during the development of PTZ induced kindling and also once kindling was established. It may be due to blockade of GABAA-ergic mechanism by both acute and chronic treatment of saffron.46,47 In another study, ethanolic and aqueous extracts decreased the duration of tonic seizures.48,49 Among the constituents of saffron extract crocetin is mainly responsible for the above pharmacological activities. In traditional medicine, the stigmas of this plant have been used as an anticonvulsant remedy.50 The aqueous and ethanolic extracts of C. sativus have shown anticonvulsant activity in PTZ and maximal electroshock seizure (MES)-induced seizures. Agents affecting the PTZ test can inhibit absence seizures. The extracts have also been shown to improve tonic clonic seizures.49 The mechanism (s) of anticonvulsant activity of the extracts is not clear. Saffron has been reported to have some behavioral effects on the central nervous system. In one study an alcoholic extract of decreased the motor activity and prolonged the sleeping time induced by hexobarbital.51 Another component of saffron, crocin did not show any effect in pentylenetetrazole-induced convulsions in mice.52
Jatamansi is a reputed Ayurvedic herb and used in various multiple formulations. jatamansi has been used in the treatment of many disease and has several activities including anticonvulsant activity, anti-parkinson’s activity, tranquillizing activity, hepatoprotective, neuroprotective etc.53 Rao et al.54 have studied ethanol extract of the roots of N. jatamansi DC for its anticonvulsant activity and neurotoxicity, alone and in combination with phenytoin in rats. The results demonstrated a significant increase in the seizure threshold by root extract against MES model as indicated by a decrease in the extension/flexion ratio. Valeranone prolonged barbiturate anesthesia, impaired rotarod performance, inhibited electroshock convulsions, and potentiated the hypothermic effects.55 Limited results from behavioral tests revealed that an extract from N. jatamansi exhibited significant antidepressant activity.56 In another study the effect of acute and sub chronic administration of alcoholic extract of the roots of N. jatamansi DC on nor epinephrine (NE), dopamine (DA), serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), GABA, and taurine on male albino Wistar rats was conducted. The acute oral administration of the extract did not change the level of NE and DA but resulted in a significant increase in the level of 5-HT and 5-HIAA. A significant increase in the level of GABA and taurine was observed in the drug-treated groups when compared to the controls. A 15-day treatment resulted in a significant increase in the levels of NE, DA, 5-HT, 5-HIAA, and GABA.57
Nutmeg (Myristica fragrans, MF) possesses anticonvulsant activity against PTZ, MES and lithium-pilocarpine induced seizures and lower doses were more effective in inhibiting seizures. The MF was without any significant effect on picrotoxin-induced convulsions and motor coordination but potentiated haloperidol induced catalepsy significantly. MF indicated signs of both CNS depression as well as stimulation. In various animal models of seizures used in study, the anticonvulsant activity of MF decreased with increasing doses. In status epilepticus, the animals receiving MF in a dose of 10 mg/kg reduced the severity of seizures at much earlier time. These observations support the biphasic effect of MF on the central nervous system. MF was without any effect on the duration of pentobarbitone-induced sleep. Though the MES test predicts activity against generalized tonic-clonic and cortical focal seizures and the PTZ test against absence seizures, the underlying neuronal abnormality is poorly understood. Diminution of brain GABA level has been reported after sub-convulsive dose of PTZ.58 Picrotoxin, the antagonist of GABA at the post-synaptic receptors, induced seizures in all the animals and its effect was not antagonized even at the dose of 100 mg/kg suggesting that GABA may not be involved in the anticonvulsant activity of MF.59,60
Nagkeshwar (Mesua ferra) is also a component of Hawan samagri. The ethanolic extract of M. ferrea flowers have been reported to reduce the duration of Hind limb tonic extension in a dose dependent manner against MES model and inhibited MES-induced convulsions. Data also showed that M. ferrea flowers significantly increased the onset time and decreased the duration of seizures by electroconvulsive shock.61 Agarwood smoke functions as endocrine disruptor and Agar wood have sedative property.62 Tagar wood Valeriana wallichii is an important component of Hawan reported to contain valepotriates and valerinic acids (with putative pharmacological activities). Root hydroethanolic extract have shown a dose dependant reduction of hind limb tonic extensor phase indicating potential antiepileptic effect on grand mal type of epilepsy in man. The extract didn’t show any adverse effects on motor coordination.63 Wood extract used for its sedative action and anticonvulsant activity64 have CNS depressant action and also have anti-convulsant effect.65
Clove is also an important part of Hawan Samagri. Clove essential oil (CEO) has been shown to significantly increase the onset of convulsion and reduce its duration in dose dependent manner compared to the control for strychnine and picrotoxin-induced convulsion. The study indicates anticonvulsant, anxiolytic and hypnotic activity of CEO. The anticonvulsant activity of a novel compound is not measured only by its ability to prevent convulsions but also to delay the onset of seizures or to reduce death rate.66 These observations also suggest that the CEO has considerable glycinergic and GABA-ergic potentiating mechanisms. Glycine and GABA are amino acids, which act as inhibitory neurotransmitters in the central nervous system and their inhibition has been implicated in convulsions. Strychnine, a potent spinal cord convulsant, blocks glycine receptors selectively to induce excitatory response in the central nervous system. Picrotoxin, on the other hand, blocks GABAA receptors to induce generalized seizures.67 The anticonvulsant action of the CEO was probably due to inhibition of the effects of strychnine and picrotoxin at glycine and GABAA receptor sites respectively. CEO has also been shown to act against neurotoxic death usually caused by chemical convulsants.68 Nagaramotha (Cyperus rotundus) is an important herb in the Ayurveda.69 Cyperotundone and α-cyperone compounds have been reported from essential oil of C. rotundus rhizomes. The effect of Cyperus esculentus and Cyperus rotundus essential oils has been reported as anticonvulsant (MES produced convulsion). The results showed dose dependent activity in the maximal electroshock (MES) induced convulsion in comparison to Diclofenac sodium.70 Nagarmotha is also known to have Iso curcumenol used as sedative.71 Nelumbo nucifera have reduced the tonic extensor convulsion induced by MES.72 The wood of Zizphus jujube is used in Hawan and the hydro-alcoholic extract of z. jujuba demonstrate the anticonvulsant effect as well as amelioration of cognitive impairment induced by seizures in rats.73 The ethanol extract of Cocos nucifera was tested for possible pharmacological effects on experimental animals. Pretreatment with extract caused significant protection against PTZ induced convulsions. The behavioral studies on mice indicate CNS depressant activity of the ethanol extract of C. nucifera. EECN potentiated significantly the duration of pentobarbital, diazepam and meprobamate induced sleep in mice, suggesting probable tranquilizing action as well as CNS depressant action.74,75 It was found to increase the brain serotonin and GABA level in mice (unpublished data). Therefore, profound analgesic and anticonvulsant activities produced by extracts may be related to the increased brain serotonin and GABA level in mice.76 The mechanism whereby extract depressed awareness, touch and pain responses, righting reflex, pinna reflex, corneal reflex, and grip strength may also be due to synapse block of the efferent pathway or by overall CNS depressant action.77 The exact chemical components responsible for such CNS depressant activity of extract are not known. Preliminary phytochemical studies revealed that it contains saponin which might be responsible for anticonvulsant properties of extract.78 The extracts also enhanced sleeping time, analgesic, and anti-convulsant activities and reduced different behavioral reflexes. In a study 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose (PGG) isolated from methanolic leaf extracts of Mangifera indica showed significant and dose-dependent inhibition of PTZ and MES-induced convulsions. Furthermore, PGG administration showed significant decrease in the locomotor activity as an indication of its CNS-depressant property; also, PGG has significantly increased the GABA levels in the cerebellum and whole brain other than the cerebellum. In conclusion, PGG isolated from M. indica showed potent anticonvulsant activity, and possible mechanism may be due to enhanced GABA levels in the brain.79
The pathogenesis of epilepsy has been strongly affected free radicals and authors have tried to hypothesize antioxidant action (Table 2) of each component of Hawan Samagri. Components of Hawan like Guggal, Saffron, Almond, Jatmansi and Coconut scavenge free radicals and hence might be helpful to stop the pathogenesis of the disease. Sesamum indicum, Sesamin is a well-known antioxidant from sesame seeds and it scavenges free radicals and significantly decreased ROS.80
Nitric Oxide is an important neurotransmitter and also related to synaptic plasticity, neuronal excitability regulation, and epileptic activity.81 NMDA glutamate receptors activate calcium release via NMDA receptor that consequently activates calcium calmoduline pathway to increase neuronal nitric oxide synthase protein expression and NO increment in brain different area. The higher NO level is able to increase the induction of generalized epilepsy. NO is known as a molecule that can easily react with O2− radicals in the brain and reduce the oxidative stress induced damage via deleting free radicals.82
It has been reported in a study that Hawan causes a reduction in NO levels in the atmosphere.83 The reduction in level of NO may be helpful in reducing the epileptic seizures. Other components of Hawan samagri have also been reported to reduce NO levels through various mechanisms (Table 2). Methanol extracts of Nardostachys jatamansi have been shown to exert inhibitory effect on nitric oxide (NO) production. The NO level decreased from 100% to 5.8% and this decreased levels could prove to have antiepileptic effect. NJ extracts down regulated iNOS in a dose-dependent manner.84 In another study saffron extract has been related to a decrease in the NO concentration.85 Lotus seed extract have been shown to possess free radical scavenging properties.86,87 Results showed that all the extracts inhibit nitric oxide accumulation and thus could be helpful in antiepileptic action. Results of a study showed that clove oil and its major constituent, eugenol, were the most active inhibitors of the nitric oxide production.88 C. rotundus rhizomes ethanolic exhibits its scavenging effect in concentration dependent manner on superoxide anion radicals, hydroxyl radicals, nitric oxide radical, hydrogen peroxide and it had a property of metal chelating and reducing power. This antioxidant activity could be helpful in preventing epilepsy.89 The methanol and EtOAc fraction of C. wightii has been shown to inhibit the NO formation by down regulation of iNOS and COX-2 gene expression.90 Guggulipid prevented the production of NO and ROS generation91 in rat astrocytoma cell line. Nishaa et al.92 and K. Kamalakara et al.93 have reported nitric oxide scavenging activity of M. ferrea pet ether and methanol extract. The biflavone and tannin fraction form Ficus racemosa bark extract has shown inhibitory action on nitric oxide and hydroxyl radicals in in-vitro studies.94 Cyperus rotundus extract suppressed the production of NO and the inhibition of NO production by the extract was due to the suppression of iNOS protein, as well as iNOS mRNA expression, determined by Western and Northern blotting analyses, respectively.95
Also, other constituents of C. rotundus including sugeonol and cyperone, could yield a modulatory effect on glutaminergic system, especially lowering the opening of NMDA receptor channels,96 which could lead to anticonvulsant effects. Ziziphus jujube (SZS) has been shown to have sedative, analgesic and antiseizure effects.97–100 NMDA-induced intracellular Ca2+ increase was almost completely abolished by SZS101 a qualitatively stronger effect than other herbs that only partially diminished the Ca2+ response. The subsequent ROS production and cell death was also reduced by SZS. Similar to RP, SZS also suppresses glutamate release and may suggest additional protection for excitotoxicity.101 Ethanol extract of Valeriana abolished cell death in NMDA-stimulated mouse cortical neurons.102 In the same study, kainate-induced cell death was marginally decreased only, suggesting the selective effect of extract on NMDA-R over other glutamate receptor subtypes.102 An inhibitory effect on glutamate binding of NMDA-R was only observed when isoborneol was present at a high concentration.103 It is therefore likely that the NMDA-R-selective cellular effects reported by Jacobo-Herrera et al. were attributed to a high concentration of isoborneol or other extract constituents yet to be identified in the ethanol extract.102 Moreover, the use of whole extract in targeting NMDA-R activity is cautioned due to the multi-faceted effect on all glutamate receptor subtypes, ionotropic and metabotropic. Evidence of the inhibition of post-synaptically located NMDA and kainite receptors by a hydro-ethanolic Crocus sativus L extract have been reported, which is partly mediated by trans-cocetin. These mechanisms contribute to the neuroprotective effect of saffron.104 Saffron has turned out to be the antagonist of postsynaptic NMDA receptors.105 Several studies have demonstrated that oxygen free radicals formed by xanthine/xanthine oxidase (X/XO) may be involved in the NMDA-mediated neurotoxicity and inhibitory action of glutamate uptake in glial cells.106–108 The results of another study showed that eugenol attenuated NMDA induced acute neurotoxicity and inhibited NMDA- induced elevation in neuronal Ca2+ uptake. Furthermore eugenol prevented acute neuronal swelling and reduced neuronal death and significantly reduced oxidative neuronal injury induced by X/XO.109 Eugenol increased the degree of INa activation and reversibly suppressed non-activating INa. In addition, at higher concentrations eugenol diminished L-type Ca+2 current and delayed rectifier K+ current. In pilocarpine-induced seizures in rats, a lower seizure severity and mortality was noted, though no shorter seizure latency effect was observed. The mechanism of action was deduced to be the synergistic blocking of INa and non-activating INa affecting neuronal spontaneous action potentials.110 Mangifera indica L. extract attenuates glutamate-induced neurotoxicity on neurons.111
Apart from the significant physical and medical applications like cleansing of the environment, curing bodily ailments and augment ing vitality and physical potentials, yagyopathy is also found to be of immense use in treatment of psychosomatic disorders and psychological and psychiatric problems. The sublimated vital elements and herbal medicines inhaled in Yagya first reach the brain and then to the lungs and other parts, the gross as well as the subtle components of the body. Thus, it has a direct healing effect on brain diseases and complexities. The body absorbs the heat of its sacrificial fire and inhales the vapors of sublimated herbs through the skin-pores and respiration. This elevated level of antioxidants upon reaching the brain and the nerves eliminates the major cause of mental tensions. The specific energy currents reduced by yagyagni and mantra shakti have significant remedial effect on the disorders and diseases ranging from headache, migraine, cold to mental dullness, intellectual deficiencies, depression, insomnia, intemperance, epilepsy, schizophrenia and varieties of manias.112
Conclusions
From the metadata analysis it seems that Hawan has been designed by the ancient scholars to fight with a plethora of diseases related to brain. As explained in text, more than 70% of the components of Hawan samagri are having a number of volatile oils that volatilize due to high temperature of fire. Most of the components have been found to be having anticonvulsant activity through one or the other mechanism. The action of maximum number of herbs is benzodiazepines, Phenobarbital, valproate like action that enhances GABA-ergic inhibition. It is quiet likely that the other volatile components those have not been explored for anticonvulsant action could add to further therapeutic antiepileptic action. The components of Hawan seem to have a multiple action in preventing epilepsy through scavenging of free radicals, increase in level of antioxidants, decrease in level of nitric oxide and other underlying mechanisms. From the pharmacological potentials of the components it can be concluded that the routine of performing Hawan might keep the threshold value of the antiepileptic elements in the body and help in preventing epilepsy however concerted efforts are required to prove the hypothesis.