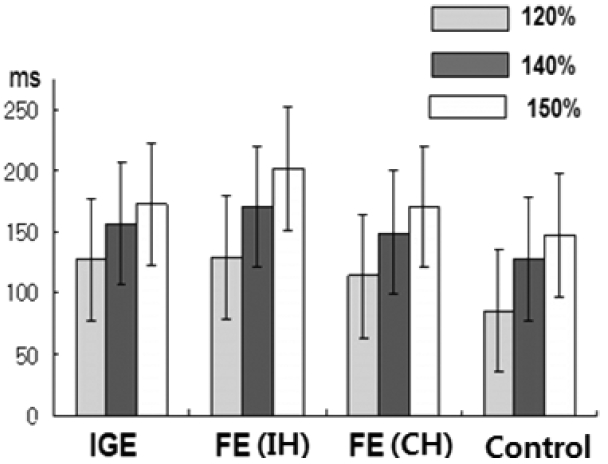
We observed that drug naïve epilepsy patients have both abnormal enhanced cortical inhibitory circuit and cortical hyperexcitability (defected intracortical inhibitory system) and we could measure this abnormal cortical inhibition and excitation balance by TMS parameters that might have lateralizing values. In this study, the CSP duration was prolonged with increasing stimulus intensity in all groups, as expected.
21,
22 A recent study also confirmed that CSP duration was affected by TMS intensity.
23 In the IGE patients, we found that the CSP duration was significantly prolonged in both hemispheres with respect to normal controls at all stimulus intensities, and no inter-hemispheric difference in CSP was found. This finding was similar to those of previous studies, including a recent meta-analysis
8,
22,
24 that suggested the increased CSP reflects a compensatory interictal mechanism, with hyperactivation of inhibitory neuronal circuits counteracting the transition from the interictal to the ictal state.
8 In contrast, in a minority of IGE patients,
10,
25 the reduced CSP could indicate that deficient inhibitory mechanisms underlying this TMS measure are involved in epileptogenesis, therefore, it is reasonable that these distinct pathophysiological substrates bear different weights in different IGE syndromes.
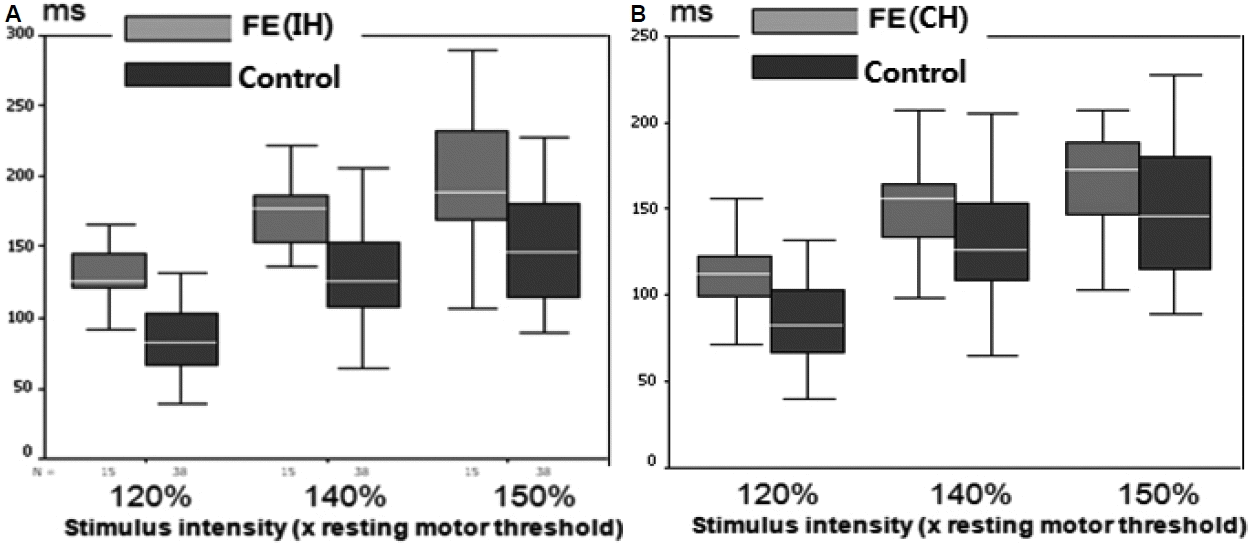
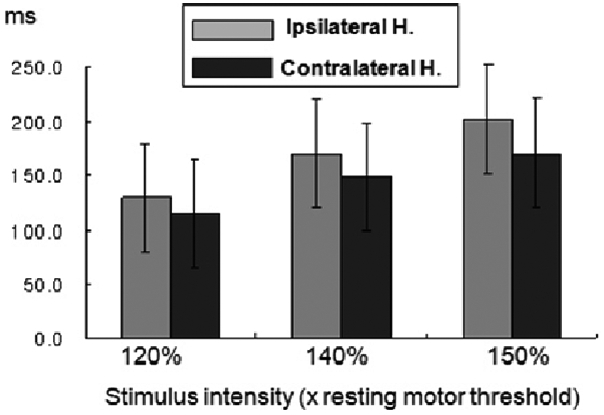
22 In FE patients, the mean CSPs were significantly longer in both the IH (to the epileptic focus) and the CH (to the epileptic focus) than in normal controls, but much longer in the IH than the CH at all intensities. These data suggest that an enhanced CSP in patients with FE reflects compensatory interictal phenomena, which may counteract seizure occurrence and the spread of epileptogenic hyperexcitability from the affected hemisphere to the contralateral one.
24 In this study, between FE and IGE patients, there was no significant difference in CSP at any stimulus intensity. However, we should consider that epilepsy syndromes can depend on different pathophysiological processes, and subsequently, the CSP (as well as other TMS measures) can be increased or reduced, depending on whether its neural substrate is involved in epileptogenesis or in interictal compensatory phenomena.
22 More prominent changes in interictal CSP in the IH of FE indicate there is a lateralized change in balance of cortical excitatory and inhibitory influences confined to the affected hemisphere and this TMS parameter could have the lateralizing value in the FE patients. Concerning CSP, the later part of silent period (> 75 ms) is ascribed to cortical inhibitory mechanisms probably related to GABA
B receptor activation.
7 In this study we observed that CSP duration was significantly prolonged in epilepsy patients and that could be related with abnormally enhanced GABA
B receptor activation. This enhanced CSP in FE patients may reflect compensatory interictal phenomena, which may counteract seizure occurrence and the spread of epileptogenic hyperexcitability. SICI and ICF mainly reflect the activation of inhibitory and excitatory cortical interneuronal circuits by a conditioning TMS pulse.
20,
26 SICI is likely to be a gamma-aminobutyric acid (GABA)-ergic effect related to GABA
A receptors, whereas ICF is a glutamatergic effect.
27–
29 In patients with cortical myoclonus
30 or JME,
9,
25,
31,
32 SICI was reduced and ICF was normal. In our IGE patients, SICI in the NDH of IGE patients was reduced, at 3 ms (
p < 0.05), and other SICI of the IGE subgroups seemed to be reduced slightly, albeit without statistical significance, which does not completely agree with previous findings.
25,
31,
33 However, this could be the result from different epilepsy syndromes. Patients with JME demonstrated significantly higher levels of cortical excitability compared with those with other types of IGE, differentiating JME from other IGE syndromes.
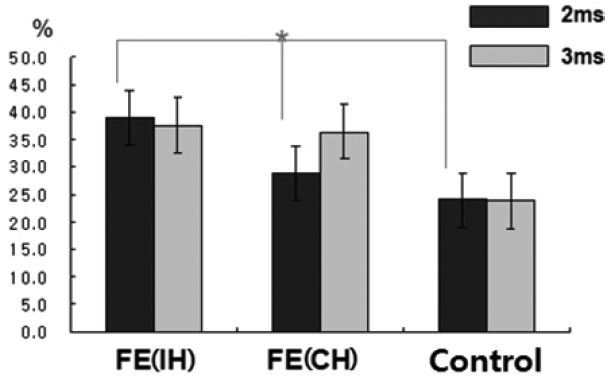
34 In patients with FE, SICI was significantly reduced in the IH (to the epileptic focus) but not the CH, which is similar to previous studies in drug-naïve patients.
9,
35 These findings suggested that the reduction in SICI was a reflection of the significantly higher excitability related to GABA
A-ergic dysfunction in the hemisphere with the epileptic focus, as compared with the contralateral side. Unlikely to CSP changes, we found reduced SICI with no difference of ICF in the IH of drug-naïve FE patients. The SICI is considered to reflect very complex inhibitory activities in the context of the primary motor cortex. The most acknowledged contribution is activation of GABA-ergic cortical inter-neurons, and particularly of GABA
A receptor-mediated effects
36 but intracortical inhibition is proportional to the dopaminergic, cholinergic and serotonergic tone as well.
35 The ICF is thought to be due to complex activation of cortical excitatory inter-neuronal circuits, among which the glutamate-related effects are the most recognized, notably mediated by the N-methyl-D-aspartate receptor.
6,
27,
29 Focal interictal spikes appear to be generated through a brief period of runaway excitation that spreads rapidly through a large local network of neurons that is terminated largely by the activation of inhibitory synaptic conductance mediated by both GABA
A and GABA
B circuits.
9,
37 Studies have shown that functionally aberrant GABA
A subunits are expressed during the early phases of epilepsy development.
38 In these conflict data, we suggested that these TMS parameters could demonstrate noninvasively the major disturbance, altered GABA-ergic function including both GABA
A and GABA
B circuits in the motor cortex. Especially in the CSP, we could find that change remains localized to the affected hemisphere in patients with focal epilepsy while is widespread and bilateral in patients with IGE. In addition, reduced SICI (MEP inhibition at short ISIs) could have the lateralizing value as well as the prolonged CSP in the FE patients.
We found that the RMT in epilepsy patients was not significantly different from that of healthy controls. We noticed a trend towards a higher RMT in epilepsy patients, but only the RMT of the NDH of the IGE group was significantly higher (
p < 0.005). Various TMS studies in epilepsy patients have reported conflicting results, ranging from a normal RMT to lower or higher RMT.
3,
10,
11,
39,
40 The RMT is believed to reflect the membrane excitability of corticospinal neurons and inter-neurons projecting onto these neurons in the motor cortex, as well as the excitability of motor neurons in the spinal cord.
27 In addition to membrane excitability itself, the RMT is related to the activity of neural inputs into pyramidal cells that affect their membrane excitability (i.e., tonic inhibitory and excitatory drives to the cortical output neurons), and provides insights into the efficacy of a chain of synapses from presynaptic cortical neurons to muscles.
41 A recent meta-analysis of the RMT in IGE reported that patients with JME have a lower RMT compared with healthy controls, but the difference was not statistically significant in other IGEs. They also reported a trend towards a higher RMT in IGE, which might represent an interictal protective mechanism against the spread or recurrence of seizures.
42 Our data were consistent with these previous reports and could also be related to the timing of the TMS study, during a seizure-free state of more than 48 h in the patients. Thus, we suppose that IGE patients might exhibit over-suppression of motor excitability due to hyper excitability of inhibitory circuits as a compensatory mechanism during an interictal state in the early stages of the disease without drug effects. While RMT mainly reflects neuronal membrane excitability, depending on ion channel conductivity, the CSP reflects the activity of intra-cortical inhibitory interneurons in the primary motor cortex, depending on GABA
B-ergic intracortical circuits.
5,
43,
44
In conclusion, we found that prolonged CSP and reduced SICI in FE indicate asymmetrically increased interictal cortical inhibition and paradoxically enhanced hyperexcitation in the epileptic hemispheres. These findings may imply a broken or altered balance of cortical inhibitory and excitatory system mainly related with altered GABA-ergic function that is a main characteristic of the epilepsy. Among several TMS parameters, CSP and SICI have a crucial role to lateralize the epileptic hemisphere in FE.